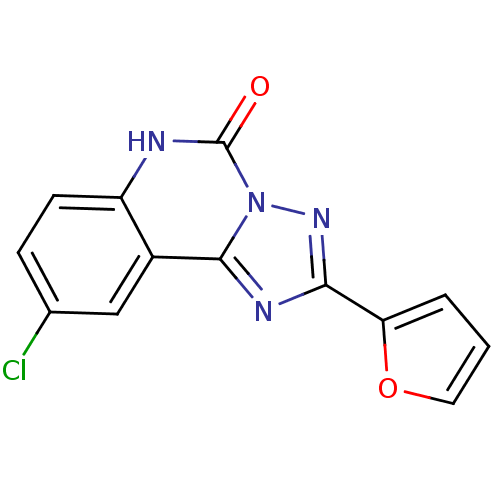
BDBM50008874 9-Chloro-2-furan-2-yl-6H-[1,2,4]triazolo[1,5-c]quinazolin-5-one::CHEMBL414023
SMILES Clc1ccc2[nH]c(=O)n3nc(nc3c2c1)-c1ccco1
InChI Key InChIKey=XEVZWKHGVYHGAM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50008874
Found 3 hits for monomerid = 50008874
Affinity DataKi: 260nMAssay Description:In vitro binding affinity at human Adenosine A3 receptor from HEK-293 cells by [125I]-AB-MECA displacement.More data for this Ligand-Target Pair
Affinity DataKi: 3.95E+3nMAssay Description:Displacement of [3H]-¿-PIA from Adenosine A1 receptor of rat cerebral cortex membranesMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Rattus norvegicus (rat))
National Institute of Diabetes
Curated by ChEMBL
National Institute of Diabetes
Curated by ChEMBL
Affinity DataKi: 4.38E+3nMAssay Description:Displacement of [3H]-CGH 21680 from Adenosine A2A receptor of rat striatal membranesMore data for this Ligand-Target Pair