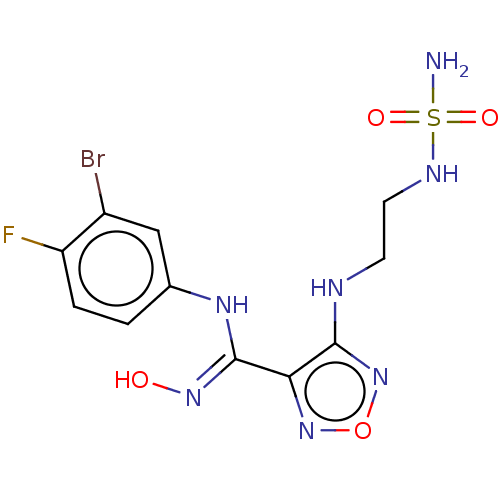
BDBM50126143 Epacadostat::INCB-024360
SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O
InChI Key InChIKey=FBKMWOJEPMPVTQ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50126143
Found 4 hits for monomerid = 50126143
TargetIndoleamine 2,3-dioxygenase 1(Homo sapiens (Human))
Kunming Institute Of Botany
Curated by ChEMBL
Kunming Institute Of Botany
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibition of recombinant human IDO1 assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate and measured after 15 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.61E+4nMAssay Description:Inhibition of recombinant human TDO assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate and measured after 15 minsMore data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Homo sapiens (Human))
Kunming Institute Of Botany
Curated by ChEMBL
Kunming Institute Of Botany
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbanc...More data for this Ligand-Target Pair
Affinity DataIC50: 1.04E+4nMAssay Description:Inhibition of TDO in human HEK293 cells assessed as reduction in kynurenine formation using L-tryptophan as substrate by absorbance based analysisMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)