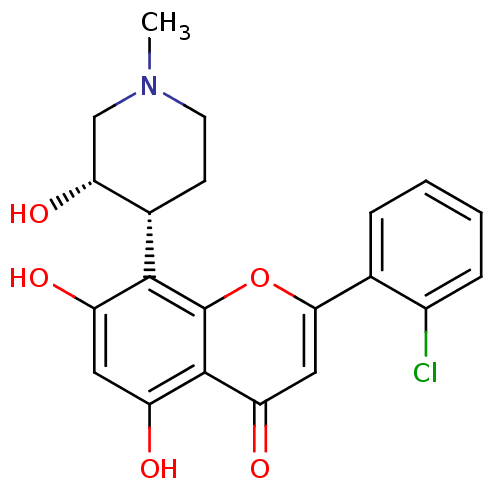
BDBM5655 2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydroxy-1-methylpiperidin-4-yl]-4H-chromen-4-one::CHEMBL428690::Flavopiridol::US10294218, Example Flavopiridol::US9617225, Flavopiridol
SMILES CN1CC[C@@H]([C@H](O)C1)c1c(O)cc(O)c2c1oc(cc2=O)-c1ccccc1Cl
InChI Key InChIKey=BIIVYFLTOXDAOV-YVEFUNNKSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 5655
Found 11 hits for monomerid = 5655
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataIC50: 30nMpH: 8.0 T: 2°CAssay Description: The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataIC50: 30nMpH: 8.0 T: 2°CAssay Description: The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataIC50: 30nMAssay Description: The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataIC50: 200nMT: 2°CAssay Description:In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP....More data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataIC50: 30nMAssay Description:Inhibition of human GST-CDK1/cyclin B1 expressed in baculovirus using [gamma-33P]ATP after 45 mins by liquid scintillation countingMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataIC50: 30nMAssay Description:Inhibition of CDK1/cyclinB (unknown origin)More data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataIC50: 27nMAssay Description:Inhibition of recombinant CDK1/cyclin B (unknown origin) using biotin-labeled histone H1 as substrate by scintillation proximity assayMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataIC50: 200nMAssay Description:Inhibition of Cyclin-dependent kinase 1-cyclin B1More data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataIC50: 30nMAssay Description:Inhibition of CDK1/cyclin B (unknown origin) in presence of [gamma-32P]ATPMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataIC50: 30nMAssay Description:Inhibition of CDK1/cyclin B1 (unknown origin) using FAM-labeled peptide and ATP as substrate preincubated for 10 mins followed by substrate addition ...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Bristol-Myers Squibb Pharmaceutical Research Institute
Affinity DataIC50: 30nMAssay Description:Inhibition of recombinant CDK1/CycB1 (unknown origin) using CTD as substrate incubated for 60 mins by Beckman scintillation counter analysisMore data for this Ligand-Target Pair