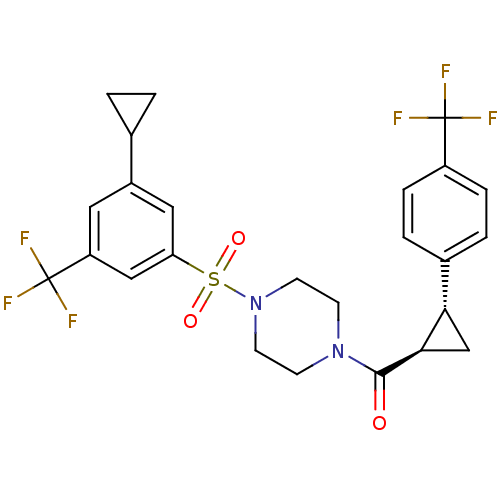
BDBM50267545 (4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfonyl)piperazin-1-yl)((1R,2R)-2-(4-(trifluoromethyl)phenyl)cyclopropyl)methanone::CHEMBL489438
SMILES FC(F)(F)c1ccc(cc1)[C@@H]1C[C@H]1C(=O)N1CCN(CC1)S(=O)(=O)c1cc(cc(c1)C(F)(F)F)C1CC1
InChI Key InChIKey=GZZYLEZVFVDDCG-FCHUYYIVSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50267545
Found 9 hits for monomerid = 50267545
Affinity DataEC50: 8nMAssay Description:Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP levelMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Displacement of [3H]CP55940 from rat recombinant CB1R expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Displacement of [3H]SR141716 from human recombinant CB1R expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 5.80nMAssay Description:Agonist activity at human recombinant CB1R expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP levelMore data for this Ligand-Target Pair
Affinity DataIC50: 9.20nMAssay Description:Antagonist activity at rat recombinant CB1R expressed in CHO cells assessed as inhibition of methanandamide-stimulated cAMP levelMore data for this Ligand-Target Pair
Affinity DataEC50: 3.40nMAssay Description:Agonist activity at rat recombinant CB1R expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP levelMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Displacement of [3H] SR141716 from rat recombinant CB1R expressed in CHO cellsMore data for this Ligand-Target Pair