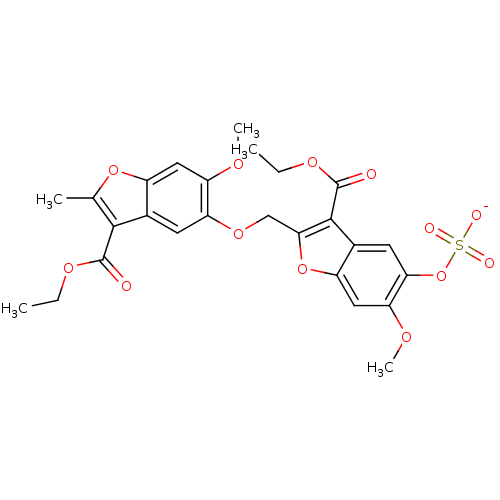
BDBM50350223 CHEMBL1812010
SMILES CCOC(=O)c1c(C)oc2cc(OC)c(OCc3oc4cc(OC)c(OS([O-])(=O)=O)cc4c3C(=O)OCC)cc12
InChI Key InChIKey=HUZCBCHTKAAOJJ-UHFFFAOYSA-M
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50350223
Found 3 hits for monomerid = 50350223
Affinity DataIC50: 6.00E+4nMAssay Description:Inhibition of human alpha-thrombin assessed as spectrozyme TH hydrolysis after 10 mins by spectrophotometric analysisChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of recombinant wild type thrombin expressed in BHK cells assessed as spectrozyme TH hydrolysis after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+5nMAssay Description:Inhibition of human plasma F10a assessed as spectrozyme TH hydrolysis after 10 minsMore data for this Ligand-Target Pair