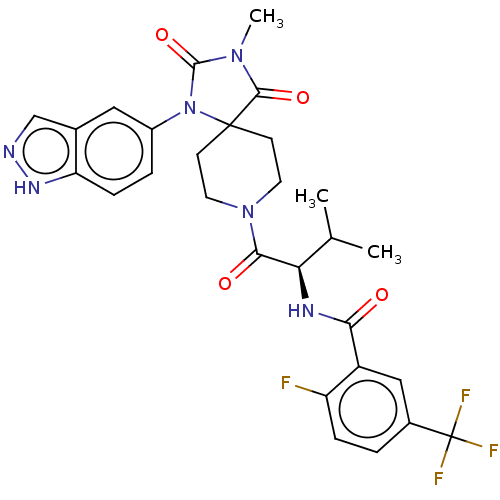
BDBM369940 (R)—N-(1-(1-(1H-indazol-5-yl)-3-methyl-2,4-dioxo-1,3,8-triazaspiro[4.5]decan-8-yl)-3-methyl-1-oxobutan-2-yl)-2-fluoro-5-(trifluoromethyl)benzamide::US10233182, Example 52
SMILES CC(C)[C@@H](NC(=O)c1cc(ccc1F)C(F)(F)F)C(=O)N1CCC2(CC1)N(C(=O)N(C)C2=O)c1ccc2[nH]ncc2c1
InChI Key
Data 15 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 15 hits for monomerid = 369940
Found 15 hits for monomerid = 369940
TargetEctonucleotide pyrophosphatase/phosphodiesterase family member 2(Homo sapiens (Human))
X-Rx
US Patent
X-Rx
US Patent
Affinity DataIC50: <500nMAssay Description:The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of LPAR3 (unknown origin)More data for this Ligand-Target Pair
TargetEctonucleotide pyrophosphatase/phosphodiesterase family member 2(Mus musculus)
X-Chem
Curated by ChEMBL
X-Chem
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of autotaxin in mouse plasma assessed as reduction in LPA (18:2) production incubated for 2 hrs LC-MS-/MS analysisMore data for this Ligand-Target Pair
TargetEctonucleotide pyrophosphatase/phosphodiesterase family member 2(Homo sapiens (Human))
X-Rx
US Patent
X-Rx
US Patent
Affinity DataIC50: 14nMAssay Description:Inhibition of autotaxin in human plasma assessed as reduction in LPA (18:2) production incubated for 2 hrs LC-MS-/MS analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
X-Chem
Curated by ChEMBL
X-Chem
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of human ERG by manual patch clamp assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
X-Chem
Curated by ChEMBL
X-Chem
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of human ERG by GLP based assayMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.04E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4.60E+4nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
TargetEctonucleotide pyrophosphatase/phosphodiesterase family member 1(Homo sapiens (Human))
X-Chem
Curated by ChEMBL
X-Chem
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of ENPP1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of LPAR1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of LPAR2 (unknown origin)More data for this Ligand-Target Pair
TargetEctonucleotide pyrophosphatase/phosphodiesterase family member 2(Homo sapiens (Human))
X-Rx
US Patent
X-Rx
US Patent
Affinity DataIC50: 55nMAssay Description:Inhibition of C-terminal FLAG-tagged human autotaxin expressed in Freestyle 293 cells assessed as reduction in choline release from LPC 16:0 pre-incu...More data for this Ligand-Target Pair