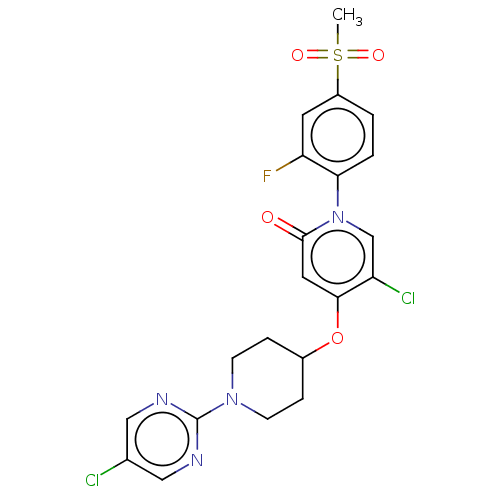
BDBM50026240 CHEMBL3338194
SMILES CS(=O)(=O)c1ccc(c(F)c1)-n1cc(Cl)c(OC2CCN(CC2)c2ncc(Cl)cn2)cc1=O
InChI Key InChIKey=OGIAVRWXUPYGGC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50026240
Found 3 hits for monomerid = 50026240
TargetGlucose-dependent insulinotropic receptor(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataEC50: 14nMAssay Description:Agonist activity at human GPR119More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of human ERG by patch clamp assayMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataEC50: >5.00E+4nMAssay Description:Activation of PXR (unknown origin)More data for this Ligand-Target Pair