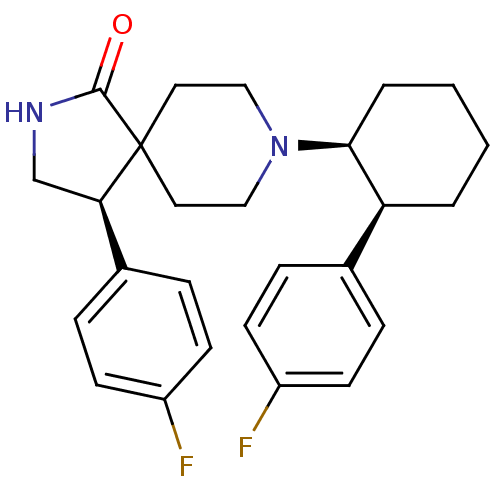
BDBM50188811 (R)-4-(4-fluoro-phenyl)-8-[(1S,2S)-2-(4-fluoro-phenyl)-cyclohexyl]-2,8-diaza-spiro[4.5]decan-1-one::CHEMBL212820
SMILES Fc1ccc(cc1)[C@H]1CNC(=O)C11CCN(CC1)[C@H]1CCCC[C@H]1c1ccc(F)cc1
InChI Key InChIKey=YUWXDNRDMDDSJC-VXNXHJTFSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50188811
Found 3 hits for monomerid = 50188811
TargetSodium- and chloride-dependent glycine transporter 1(Homo sapiens (Human))
F. Hoffmann-La Roche
Curated by ChEMBL
F. Hoffmann-La Roche
Curated by ChEMBL
Affinity DataEC50: 1.30E+3nMAssay Description:Inhibition of [3H]glycine uptake at GlyT1More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Displacement of [3H]naloxone from mu opioid receptor expressed in BHK cellsMore data for this Ligand-Target Pair
TargetSodium- and chloride-dependent glycine transporter 2(Homo sapiens (Human))
F. Hoffmann-La Roche
Curated by ChEMBL
F. Hoffmann-La Roche
Curated by ChEMBL
Affinity DataEC50: 8.36E+3nMAssay Description:Inhibition of [3H]glycine uptake at GlyT2More data for this Ligand-Target Pair