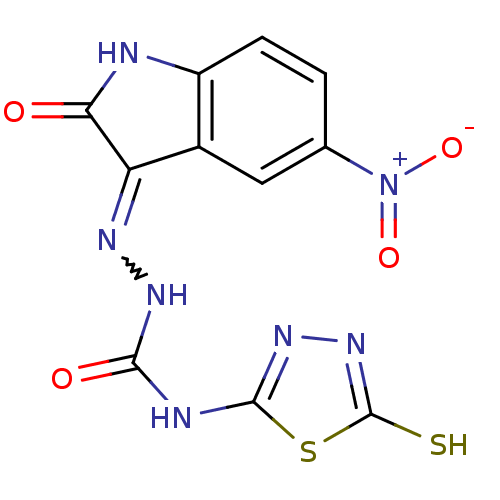
BDBM50221270 4-(4,5-dihydro-5-thioxo-1,3,4-thiadiazol-2-yl)-1-(5-nitro-2-oxoindolin-3-ylidene)semicarbazide::CHEMBL236146
SMILES [O-][N+](=O)c1ccc2NC(=O)C(=NNC(=O)Nc3nnc(S)s3)c2c1
InChI Key InChIKey=GGKRHRIXTQNXAZ-UHFFFAOYSA-N
Data 3 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50221270
Found 3 hits for monomerid = 50221270
Affinity DataKi: 1.25E+3nMAssay Description:Inhibition of catalytic domain of human cloned carbonic anhydrase9 by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 2.15E+3nMAssay Description:Inhibition of human cloned carbonic anhydrase2 by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 5.95E+3nMAssay Description:Inhibition of human cloned carbonic anhydrase1 by CO2 hydration methodMore data for this Ligand-Target Pair