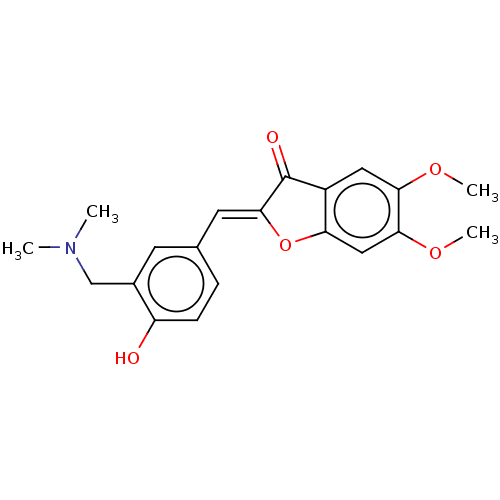
BDBM50234823 CHEMBL4069184
SMILES COc1cc2O\C(=C/c3ccc(O)c(CN(C)C)c3)C(=O)c2cc1OC
InChI Key InChIKey=SDHJLISGUOUYEM-UWVJOHFNSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50234823
Found 3 hits for monomerid = 50234823
Affinity DataIC50: 127nMAssay Description:Inhibition of AChE in rat brain cortex homogenates using acetylthiocholine iodide as substrate in presence of BuChE inhibitor tetraisopropyl pyrophos...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Sichuan University
Curated by ChEMBL
Sichuan University
Curated by ChEMBL
Affinity DataIC50: 411nMAssay Description:Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 510nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate measured after 15 mins by Ellman's methodMore data for this Ligand-Target Pair