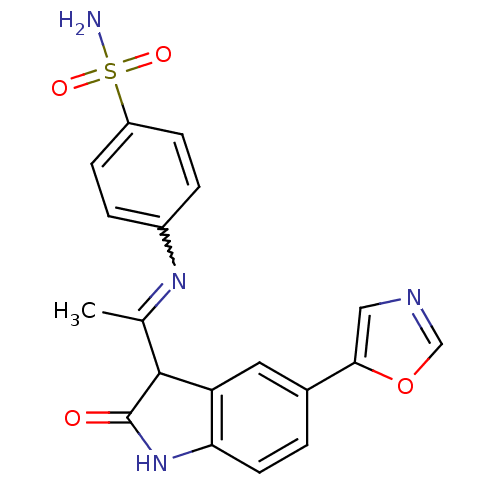
BDBM7688 4-({1-[(3Z)-5-(1,3-oxazol-5-yl)-2-oxo-2,3-dihydro-1H-indol-3-ylidene]ethyl}amino)benzene-1-sulfonamide::4-[1-(5-Oxazol-5-yl-2-oxo-1,2-dihydro-indol-3-ylidene)-ethylamino]benzenesulfonamide::Oxindole-Based Inhibitor 24
SMILES CC(=Nc1ccc(cc1)S(N)(=O)=O)C1C(=O)Nc2ccc(cc12)-c1cnco1
InChI Key InChIKey=OPLHNOVRWYFVGR-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 7688
Found 4 hits for monomerid = 7688
Affinity DataIC50: 7.10nMpH: 7.5 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence 1.4 uM ATP/[gamma-32P] ATP. A...More data for this Ligand-Target Pair