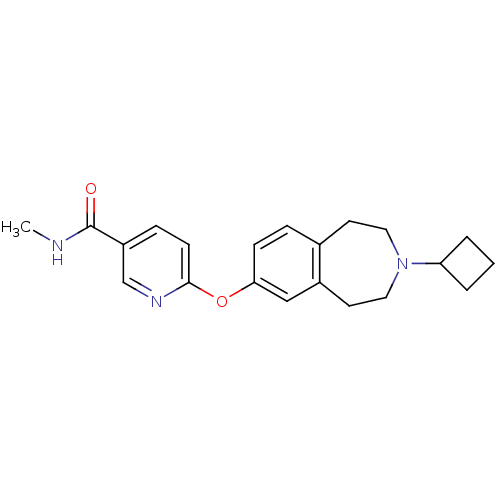
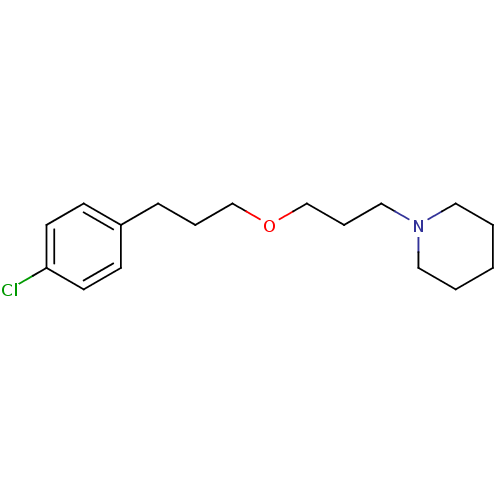
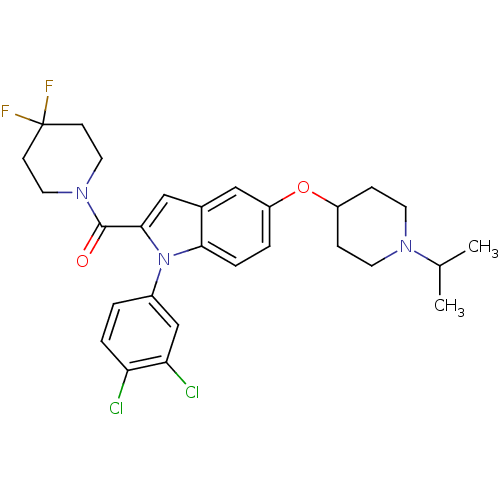
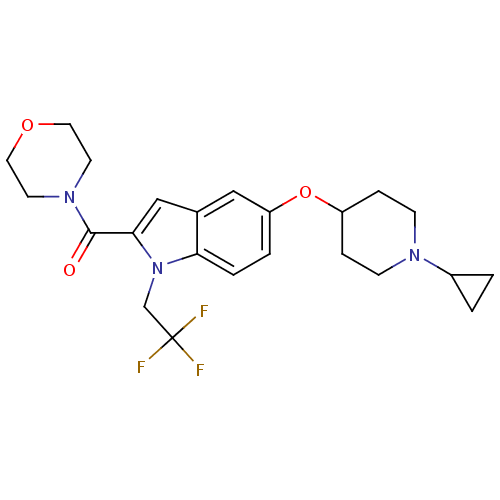
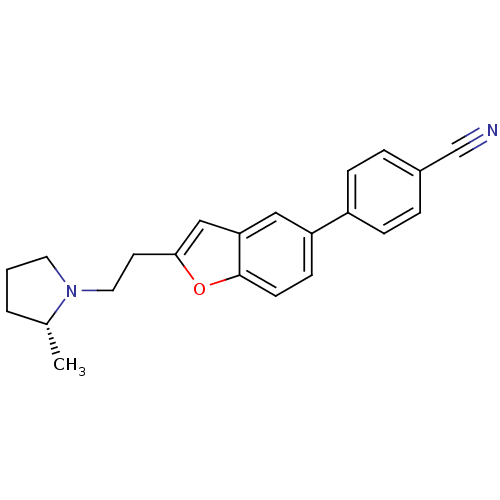
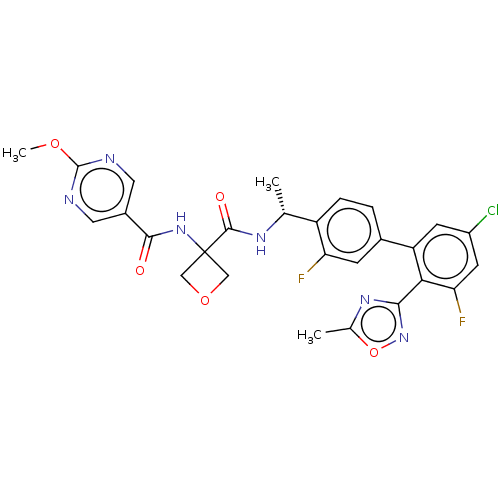
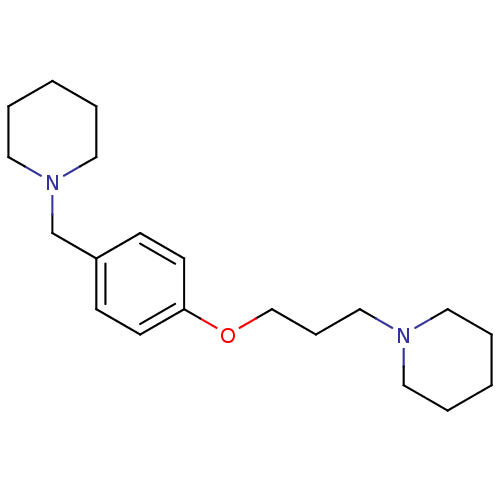
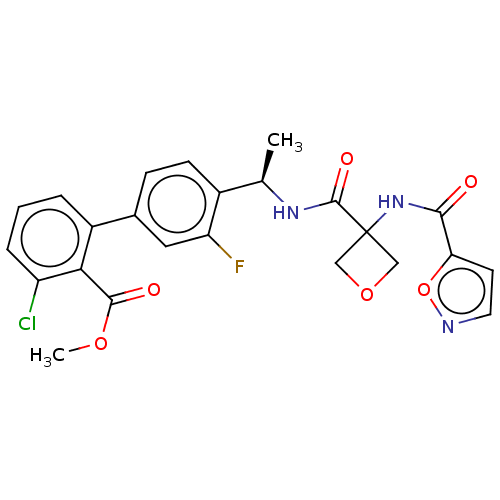
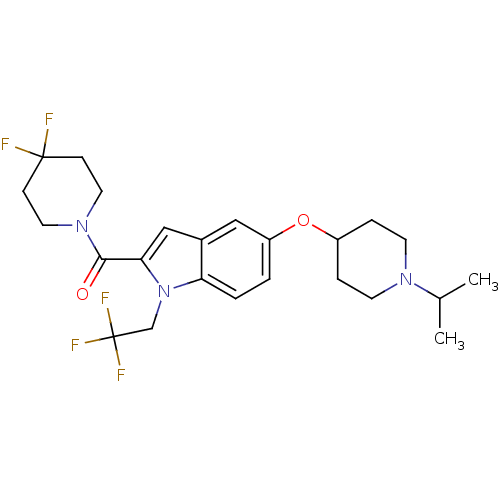
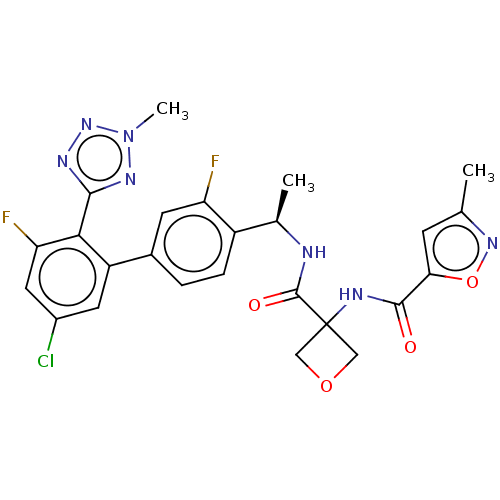
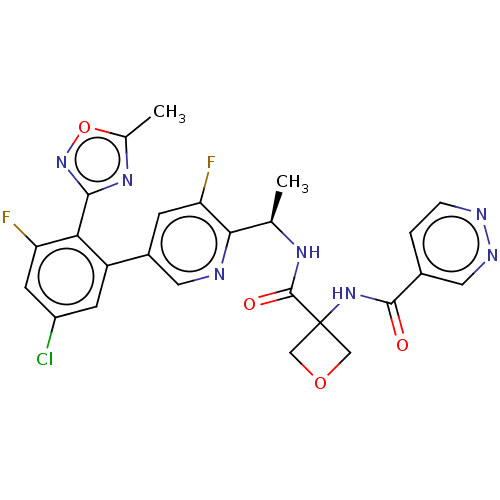
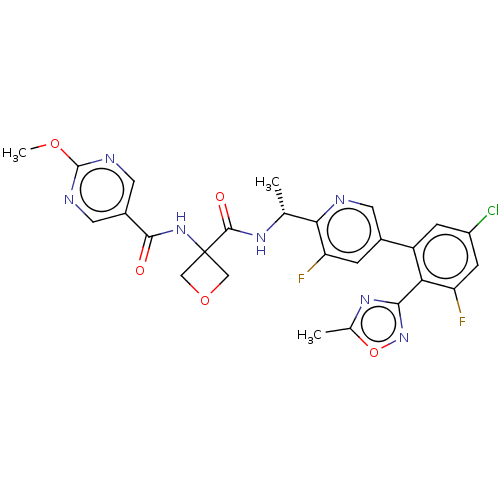
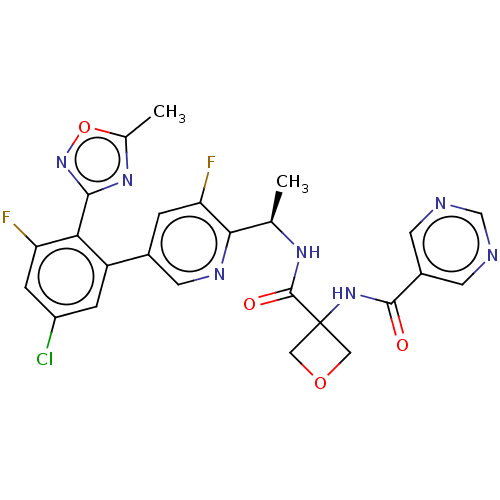
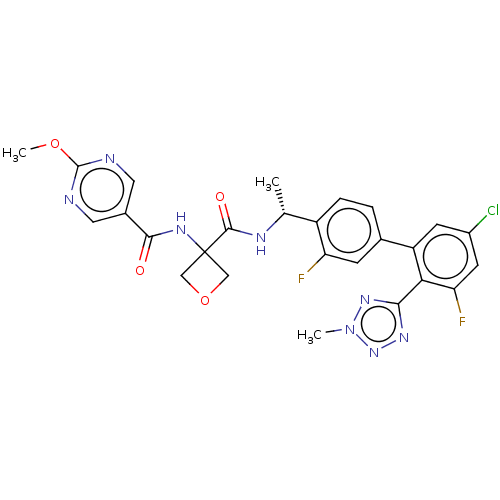
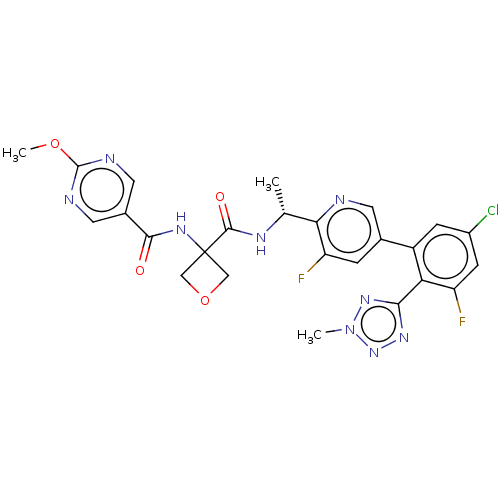
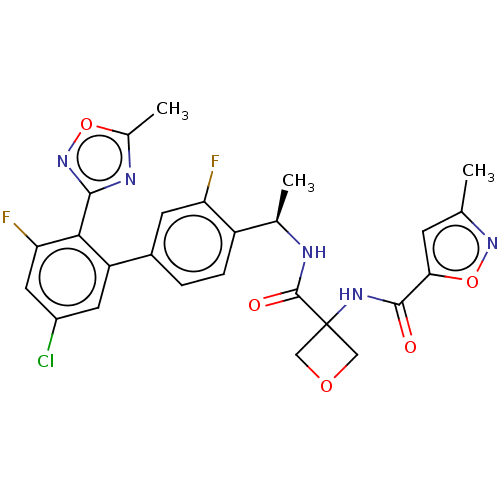
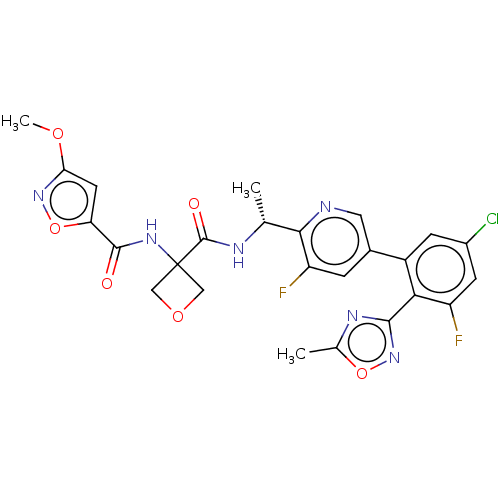
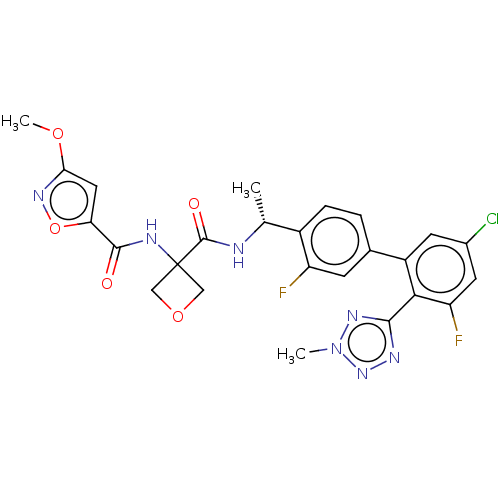
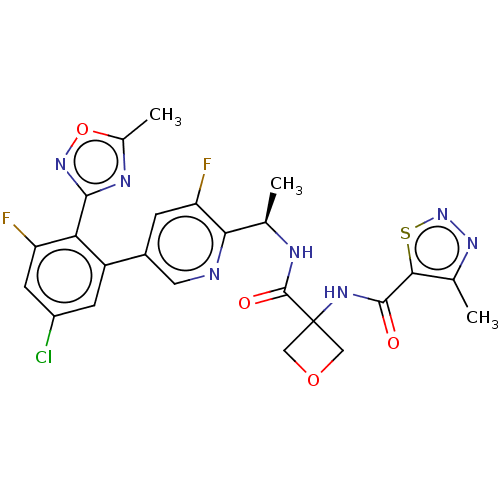
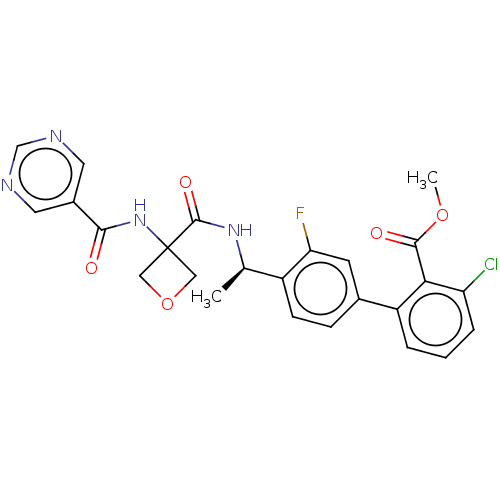
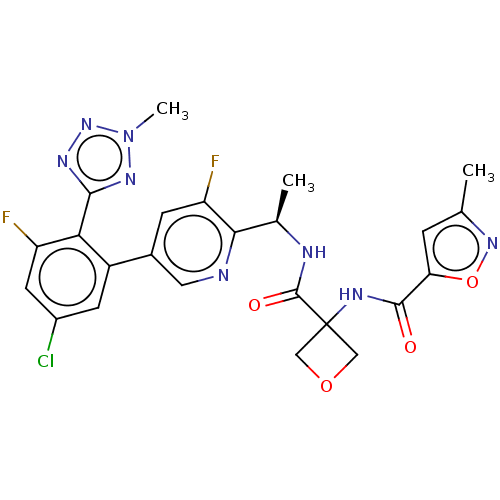
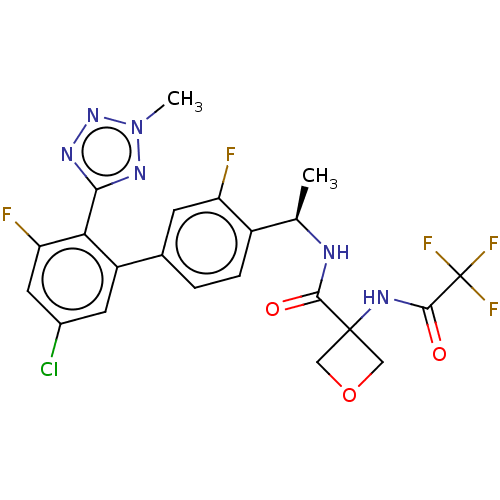
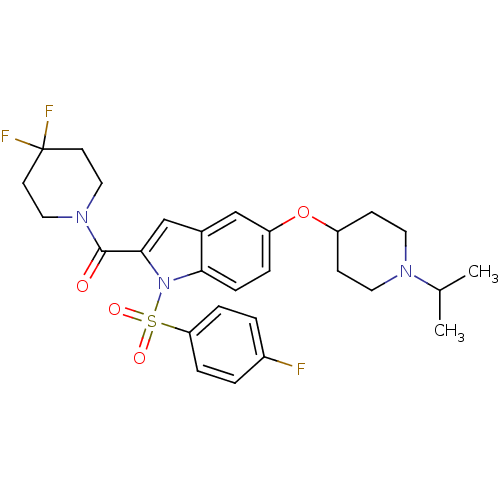
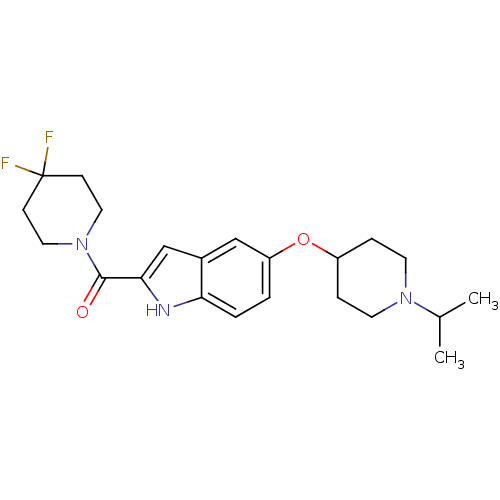
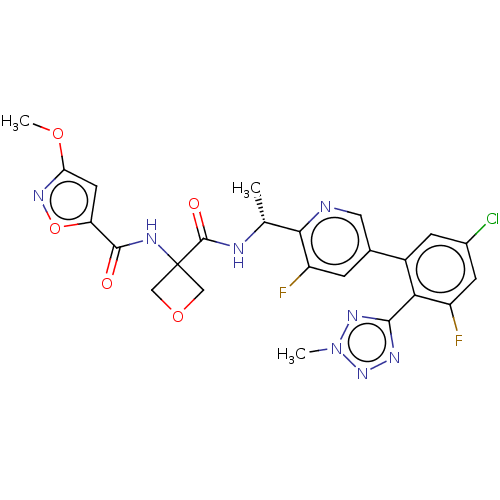
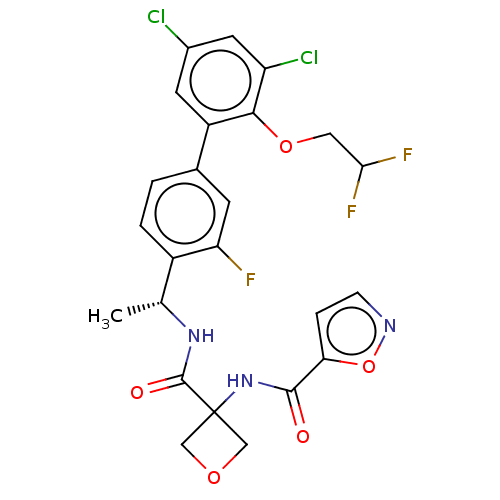
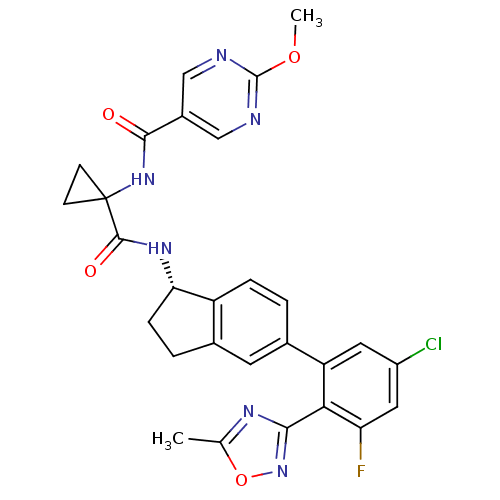
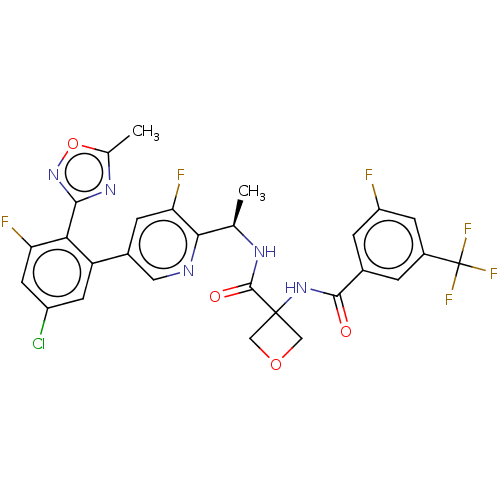
Affinity DataKi: 0.120nMAssay Description:Binding affinity to histamine H3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Antagonist activity at human histamine H3 receptor expressed in HEK293 cells by [35S]gammaS binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Binding affinity to histamine H3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Binding affinity to histamine H3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 1nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 1nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 1.10nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 1.10nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 1.10nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 1.10nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 1.30nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 1.40nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 1.40nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 1.40nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 1.70nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Binding affinity to histamine H3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Binding affinity to histamine H3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.20nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 2.30nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 2.40nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 2.5nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 2.60nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Binding assay using Bradykinin-1 receptor.More data for this Ligand-Target Pair
Affinity DataKi: 2.60nMpH: 7.4Assay Description:For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding assay using Bradykinin-1 receptor.More data for this Ligand-Target Pair