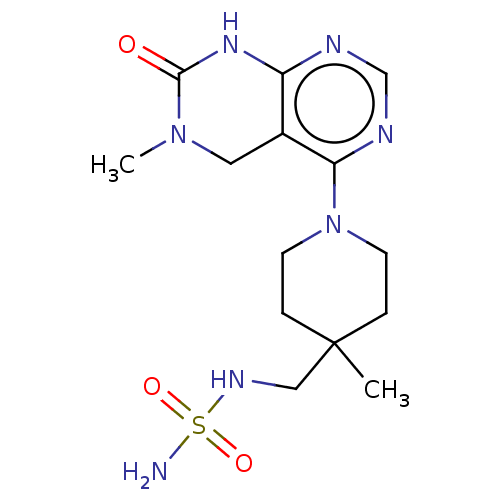
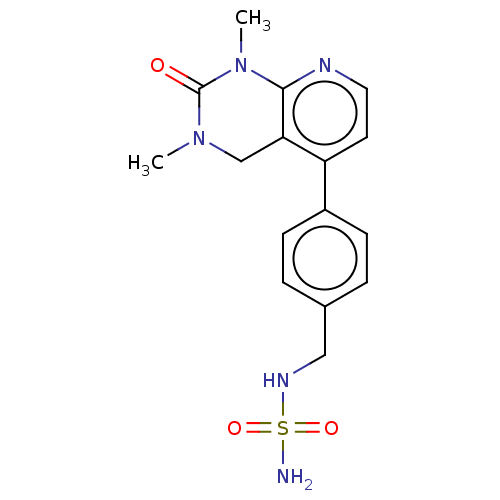
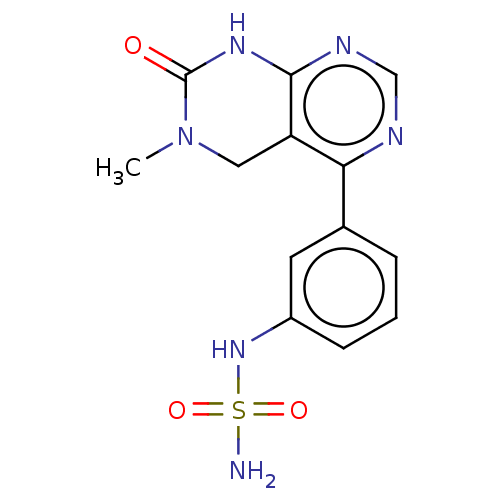
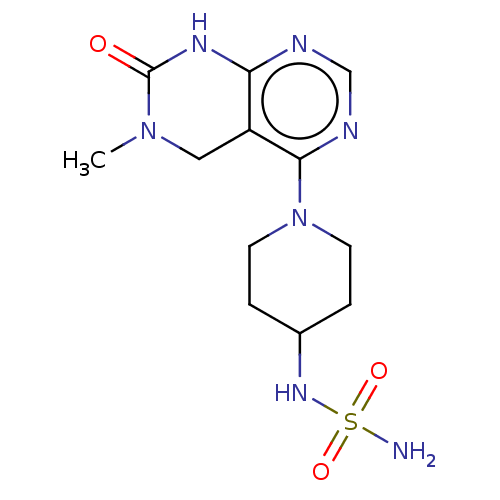
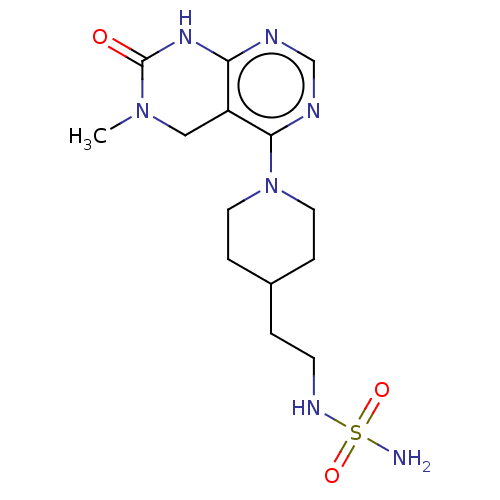
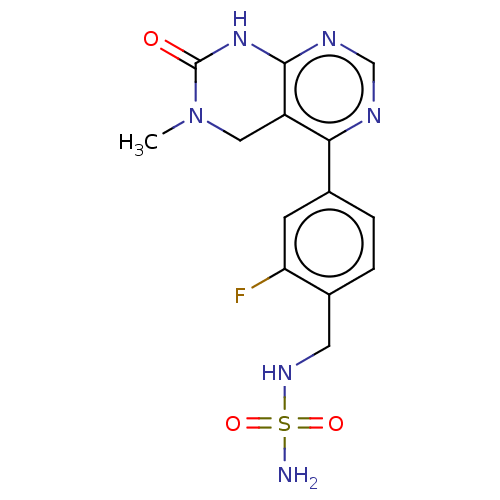
TargetFatty acid-binding protein, adipocyte(Homo sapiens (Human))
University Of Minnesota
Curated by ChEMBL
University Of Minnesota
Curated by ChEMBL
Affinity DataKi: 670nMAssay Description:Displacement of radiolabeled 1-anilinonapthalene 8-sulfonic acid from AFABP expressed in Escherichia coli BL21 (DE3) by fluorescence spectrophotometr...More data for this Ligand-Target Pair
Affinity DataKi: 3.40E+3nMAssay Description:Displacement of radiolabeled 1-anilinonapthalene 8-sulfonic acid from EFABP expressed in Escherichia coli BL21 (DE3) by fluorescence spectrophotometr...More data for this Ligand-Target Pair
TargetFatty acid-binding protein, intestinal(Homo sapiens (Human))
University Of Minnesota
Curated by ChEMBL
University Of Minnesota
Curated by ChEMBL
Affinity DataKi: 6.57E+3nMAssay Description:Displacement of radiolabeled 1-anilinonapthalene 8-sulfonic acid from IFABP expressed in Escherichia coli BL21 (DE3) by fluorescence spectrophotometr...More data for this Ligand-Target Pair
TargetFatty acid-binding protein, liver(Homo sapiens (Human))
University Of Minnesota
Curated by ChEMBL
University Of Minnesota
Curated by ChEMBL
Affinity DataKi: 8.17E+3nMAssay Description:Displacement of radiolabeled 1-anilinonapthalene 8-sulfonic acid from LFABP expressed in Escherichia coli BL21 (DE3) by fluorescence spectrophotometr...More data for this Ligand-Target Pair
TargetFatty acid-binding protein, heart(Homo sapiens (Human))
University Of Minnesota
Curated by ChEMBL
University Of Minnesota
Curated by ChEMBL
Affinity DataKi: 9.07E+3nMAssay Description:Displacement of radiolabeled 1-anilinonapthalene 8-sulfonic acid from HFABP expressed in Escherichia coli BL21 (DE3) by fluorescence spectrophotometr...More data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:Inhibition of ENPP1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Inhibition of ENPP1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Inhibition of ENPP1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 48nMAssay Description:Inhibition of ENPP1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 164nMAssay Description:Inhibition of ENPP1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 171nMAssay Description:Inhibition of ENPP1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 236nMAssay Description:Inhibition of ENPP1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 326nMAssay Description:Inhibition of ENPP1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 360nMAssay Description:Inhibition of ENPP1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 389nMAssay Description:Inhibition of ENPP1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 452nMAssay Description:Inhibition of ENPP1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 525nMAssay Description:Inhibition of ENPP1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 669nMAssay Description:Inhibition of ENPP1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 759nMAssay Description:Inhibition of ENPP1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 766nMAssay Description:Inhibition of ENPP1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 773nMAssay Description:Inhibition of ENPP1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 916nMAssay Description:Inhibition of ENPP1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:ENPP1 hydrolyzes nucleotides or nucleotide derivatives to produce nucleoside -5′-monophosphate and pyrophosphate. in addition, ENPP1 hydrolyzes...More data for this Ligand-Target Pair