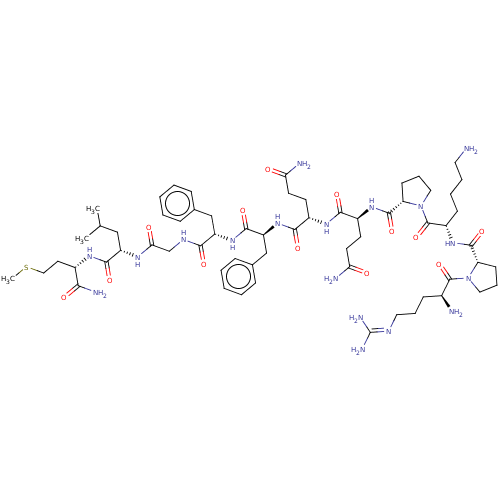
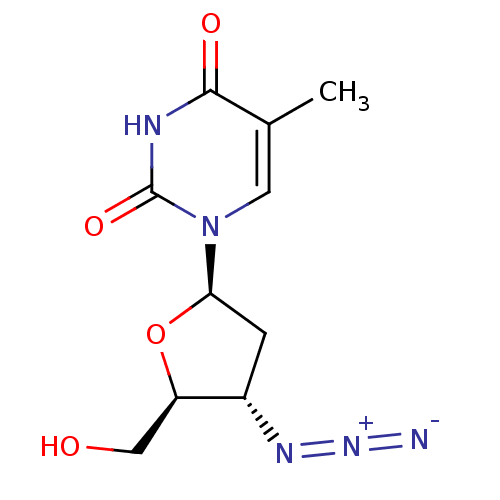
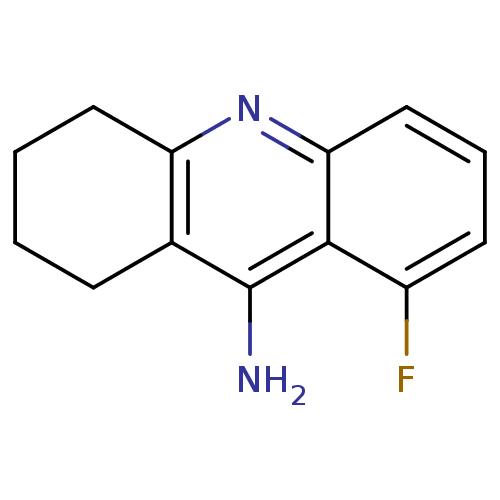
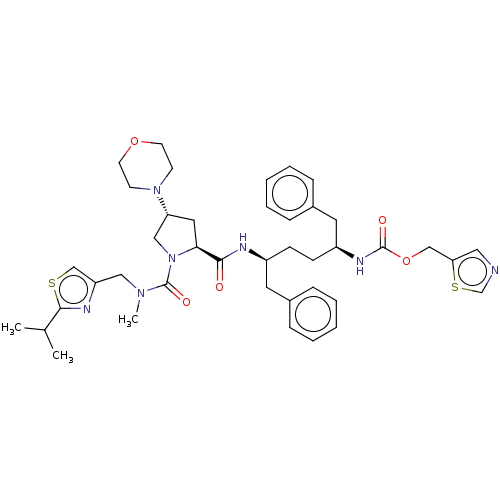
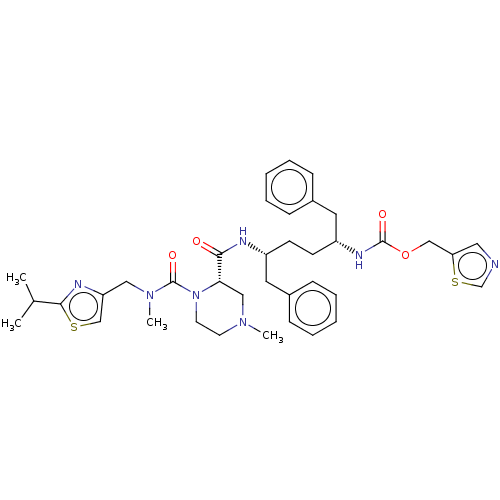
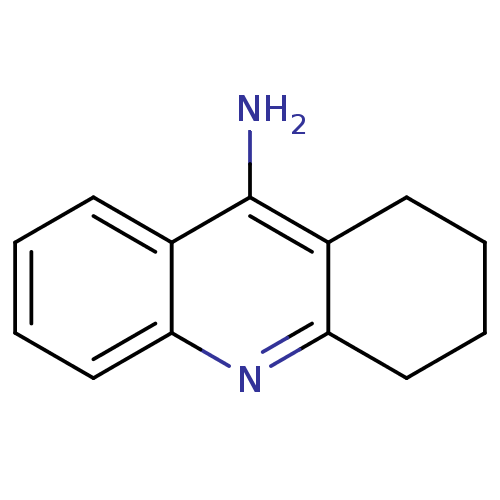
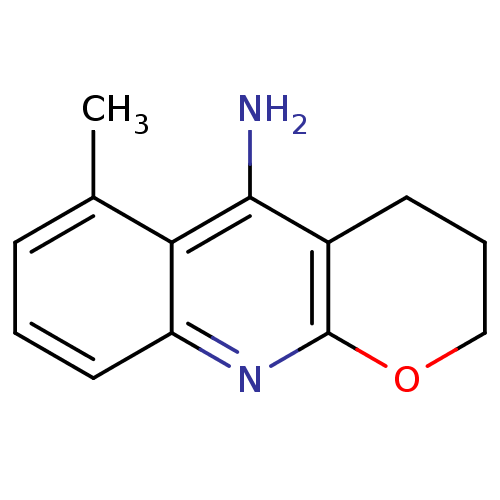
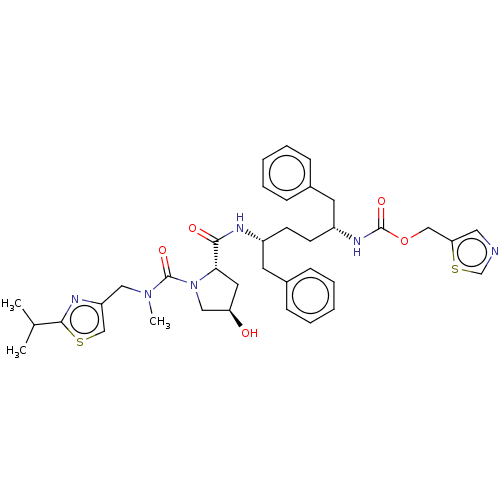
Affinity DataKi: 0.170nMAssay Description:Evaluated for the binding affinity towards NK1 receptor in the striatal membranes of guinea pigMore data for this Ligand-Target Pair
Affinity DataKi: 0.170nMAssay Description:In vitro binding affinity against substance P (NK-1) receptor in human IM-9 cell using [125I]-BH-SPMore data for this Ligand-Target Pair
Affinity DataKi: 0.170nMAssay Description:Evaluated for the binding affinity towards NK1 receptor in the striatal membranes of guinea pigMore data for this Ligand-Target Pair
Affinity DataKi: 0.480nMAssay Description:In vitro binding affinity for the Tachykinin receptor 1 in human IM-9 cell using [125I]-BH-SP of the compound.More data for this Ligand-Target Pair
Affinity DataKi: 0.480nMAssay Description:In vitro binding affinity for the Tachykinin receptor 1 in human IM-9 cell using [125I]-BH-SP of the compound.More data for this Ligand-Target Pair
Affinity DataKi: 0.660nMAssay Description:In vitro binding affinity for the Tachykinin receptor 1 in human IM-9 cell using [125I]-BH-SP of the compound.More data for this Ligand-Target Pair
Affinity DataKi: 0.660nMAssay Description:In vitro binding affinity for the Tachykinin receptor 1 in human IM-9 cell using [125I]-BH-SP of the compound.More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:In vitro binding affinity of the compound towards human NK-1 receptor in IM-9 cells using [3H]-SP of substance P antagonistMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:In vitro binding affinity against substance P (NK-1) receptor in human IM-9 cell using [125I]-BH-SPMore data for this Ligand-Target Pair
Affinity DataKi: 2.40nMAssay Description:In vitro binding affinity of the compound towards human NK-1 receptor in IM-9 cells using [3H]-SP of substance P antagonistMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Evaluated for the binding affinity against NK1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 49nMAssay Description:In vitro binding affinity for the Tachykinin receptor 1 in human IM-9 cell using [125I]-BH-SP of the compound.More data for this Ligand-Target Pair
Affinity DataKi: 51nMAssay Description:Evaluated for the binding affinity against NK1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3.70E+3nMAssay Description:Evaluated for the binding affinity against NK2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:In vitro binding affinity for the Tachykinin receptor 1 in human IM-9 cell using [125I]BH-SP of the compound.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:In vitro binding affinity against substance P (NK-1) receptor in human IM-9 cell using [125I]-BH-SPMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Tested for inhibitory activity against substance P receptor.More data for this Ligand-Target Pair
Affinity DataIC50: 39nMAssay Description:In vitro inhibition of acetylcholinesterase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an...More data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:In vitro inhibition of acetylcholinesterase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an...More data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition on HIV1 reverse transcriptase p66/p51More data for this Ligand-Target Pair
Affinity DataIC50: 78nMAssay Description:In vitro inhibition of acetylcholinesterase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataIC50: 94nMAssay Description:The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an...More data for this Ligand-Target Pair
Affinity DataIC50: 94nMAssay Description:The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an...More data for this Ligand-Target Pair
Affinity DataIC50: 94nMAssay Description:The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an...More data for this Ligand-Target Pair
Affinity DataIC50: 99nMAssay Description:In vitro inhibition of acetylcholinesterase isolated from human erythrocytes.More data for this Ligand-Target Pair
TargetSodium channel protein type 5 subunit alpha(Homo sapiens (Human))
Gilead Sciences
Curated by ChEMBL
Gilead Sciences
Curated by ChEMBL
Affinity DataIC50: <100nMAssay Description:Inhibition of NaV1.5 expressed in human HEK293 cells assessed as inhibition of late sodium current at 3 Hz frequency by manual patch clamp techniqueMore data for this Ligand-Target Pair
TargetSodium channel protein type 5 subunit alpha(Homo sapiens (Human))
Gilead Sciences
Curated by ChEMBL
Gilead Sciences
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of NaV1.5 expressed in human HEK293 cells assessed as inhibition of late sodium current at -80 mV resting membrane potential by electrophy...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMT: 2°CAssay Description:The potencies of the compounds as inhibitors of human hepatic cytochromes P450 of the CYP3A subfamily (particularly CYP3A4) were assessed using well-...More data for this Ligand-Target Pair
Affinity DataIC50: <100nMT: 2°CAssay Description:The potencies of the compounds as inhibitors of human hepatic cytochromes P450 of the CYP3A subfamily (particularly CYP3A4) were assessed using well-...More data for this Ligand-Target Pair
Affinity DataIC50: 110nMT: 2°CAssay Description:The potencies of the compounds as inhibitors of human hepatic cytochromes P450 of the CYP3A subfamily (particularly CYP3A4) were assessed using well-...More data for this Ligand-Target Pair
Affinity DataIC50: 120nMT: 2°CAssay Description:The potencies of the compounds as inhibitors of human hepatic cytochromes P450 of the CYP3A subfamily (particularly CYP3A4) were assessed using well-...More data for this Ligand-Target Pair
Affinity DataIC50: 120nMT: 2°CAssay Description:The potencies of the compounds as inhibitors of human hepatic cytochromes P450 of the CYP3A subfamily (particularly CYP3A4) were assessed using well-...More data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibition on HIV1 reverse transcriptase p66/p51More data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibition on HIV1 reverse transcriptase p66/p51More data for this Ligand-Target Pair
Affinity DataIC50: 130nMT: 2°CAssay Description:The potencies of the compounds as inhibitors of human hepatic cytochromes P450 of the CYP3A subfamily (particularly CYP3A4) were assessed using well-...More data for this Ligand-Target Pair
Affinity DataIC50: 137nMAssay Description:The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an...More data for this Ligand-Target Pair
Affinity DataIC50: 137nMAssay Description:The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an...More data for this Ligand-Target Pair
Affinity DataIC50: 137nMAssay Description:The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an...More data for this Ligand-Target Pair
Affinity DataIC50: 140nMT: 2°CAssay Description:The potencies of the compounds as inhibitors of human hepatic cytochromes P450 of the CYP3A subfamily (particularly CYP3A4) were assessed using well-...More data for this Ligand-Target Pair
Affinity DataIC50: 143nMAssay Description:In vitro inhibition of acetylcholinesterase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataIC50: 150nMT: 2°CAssay Description:The potencies of the compounds as inhibitors of human hepatic cytochromes P450 of the CYP3A subfamily (particularly CYP3A4) were assessed using well-...More data for this Ligand-Target Pair
Affinity DataIC50: 150nMT: 2°CAssay Description:The potencies of the compounds as inhibitors of human hepatic cytochromes P450 of the CYP3A subfamily (particularly CYP3A4) were assessed using well-...More data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:In vitro inhibition of acetylcholinesterase isolated from human erythrocytes.More data for this Ligand-Target Pair
Affinity DataIC50: 150nMT: 2°CAssay Description:The potencies of the compounds as inhibitors of human hepatic cytochromes P450 of the CYP3A subfamily (particularly CYP3A4) were assessed using well-...More data for this Ligand-Target Pair
Affinity DataIC50: 160nMT: 2°CAssay Description:The potencies of the compounds as inhibitors of human hepatic cytochromes P450 of the CYP3A subfamily (particularly CYP3A4) were assessed using well-...More data for this Ligand-Target Pair
Affinity DataIC50: 160nMT: 2°CAssay Description:The potencies of the compounds as inhibitors of human hepatic cytochromes P450 of the CYP3A subfamily (particularly CYP3A4) were assessed using well-...More data for this Ligand-Target Pair
Affinity DataIC50: 170nMT: 2°CAssay Description:The potencies of the compounds as inhibitors of human hepatic cytochromes P450 of the CYP3A subfamily (particularly CYP3A4) were assessed using well-...More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)