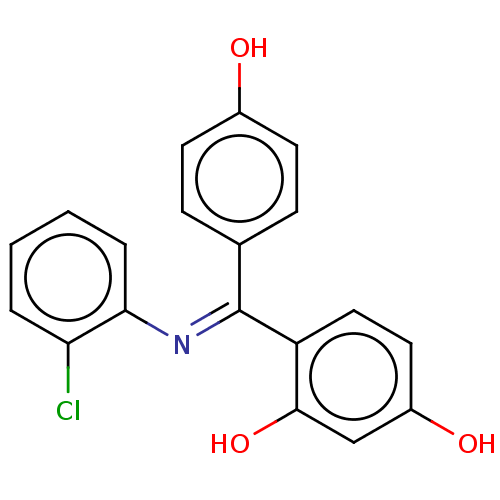
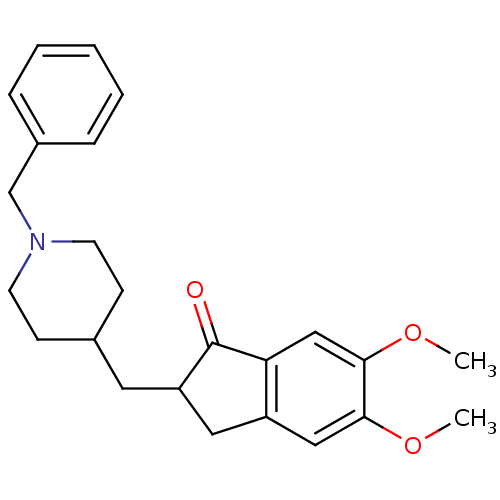
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1/2(Rattus norvegicus)
Chinese Academy Of Science
Curated by ChEMBL
Chinese Academy Of Science
Curated by ChEMBL
Affinity DataKi: 18nMAssay Description:Ability to inhibit Steroid 5-alpha-reductase in rat using Enzyme kinetics method.More data for this Ligand-Target Pair
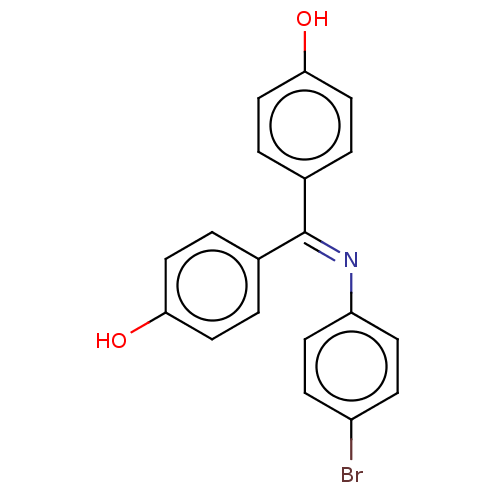
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1/2(Rattus norvegicus)
Chinese Academy Of Science
Curated by ChEMBL
Chinese Academy Of Science
Curated by ChEMBL
Affinity DataKi: 46nMAssay Description:Ability to inhibit Steroid 5-alpha-reductase in rat using Enzyme kinetics method.More data for this Ligand-Target Pair
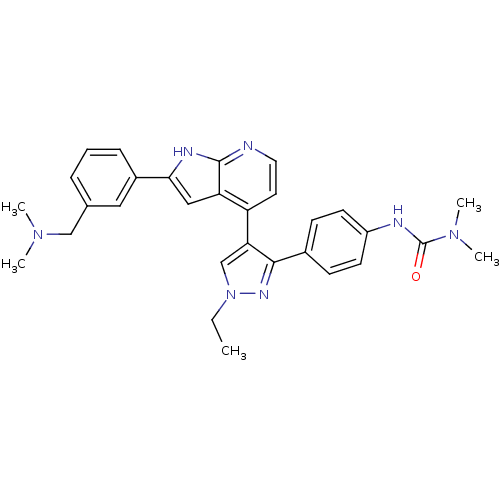
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1/2(Rattus norvegicus)
Chinese Academy Of Science
Curated by ChEMBL
Chinese Academy Of Science
Curated by ChEMBL
Affinity DataKi: 55nMAssay Description:Ability to inhibit Steroid 5-alpha-reductase in rat using Enzyme kinetics method.More data for this Ligand-Target Pair
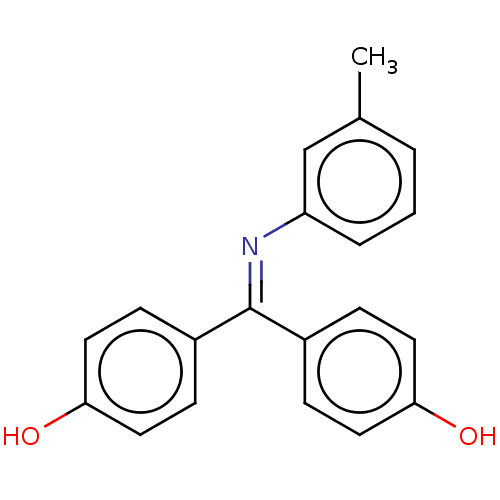
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1/2(Rattus norvegicus)
Chinese Academy Of Science
Curated by ChEMBL
Chinese Academy Of Science
Curated by ChEMBL
Affinity DataKi: 69nMAssay Description:Ability to inhibit Steroid 5-alpha-reductase in rat using Isotope method [3H]T to [3H]-DHT]More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1/2(Rattus norvegicus)
Chinese Academy Of Science
Curated by ChEMBL
Chinese Academy Of Science
Curated by ChEMBL
Affinity DataKi: 88nMAssay Description:Ability to inhibit Steroid 5-alpha-reductase in rat using Isotope method [3H]T to [3H]-DHT]More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1/2(Rattus norvegicus)
Chinese Academy Of Science
Curated by ChEMBL
Chinese Academy Of Science
Curated by ChEMBL
Affinity DataKi: 100nMAssay Description:Ability to inhibit Steroid 5-alpha-reductase in rat using Isotope method [3H]T to [3H]-DHT]More data for this Ligand-Target Pair
Affinity DataKi: 232nMAssay Description:Noncompetitive inhibition of equine serum BChE pre-incubated for 5 mins before butyrylthiocholine iodide substrate addition and measured after 2 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti...More data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti...More data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti...More data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti...More data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of aurora B assessed as inhibition of histone H3 phosphorylation by ELISA based cellular mechanistic assayMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti...More data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti...More data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Relative binding affinity for human estrogen receptor alpha by displacement of [3H]-estradiolMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 24nMAssay Description:Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti...More data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 28nMAssay Description:Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair
TargetKelch-like ECH-associated protein 1(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 31nMAssay Description:Binding affinity to Keap1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Inhibition of aurora B assessed as inhibition of histone H3 phosphorylation by ELISA based cellular mechanistic assayMore data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 33nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 41nMAssay Description:Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti...More data for this Ligand-Target Pair
Affinity DataIC50: 44nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 45nMAssay Description:Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 2 mins followed by substrate addition and measured after...More data for this Ligand-Target Pair
Affinity DataIC50: 51nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 51nMAssay Description:Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti...More data for this Ligand-Target Pair
Affinity DataIC50: 51nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 67nMAssay Description:Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti...More data for this Ligand-Target Pair
Affinity DataIC50: 67nMAssay Description:Inhibition of aurora B assessed as inhibition of histone H3 phosphorylation by ELISA based cellular mechanistic assayMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 83nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory Of Combinatorial Biosynthesis And Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti...More data for this Ligand-Target Pair
Affinity DataIC50: 104nMAssay Description:Inhibition of aurora B assessed as inhibition of histone H3 phosphorylation by ELISA based cellular mechanistic assayMore data for this Ligand-Target Pair
Affinity DataIC50: 108nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair
TargetNuclear factor erythroid 2-related factor 2(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 115nMAssay Description:Inhibition of Nrf2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 124nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured afte...More data for this Ligand-Target Pair

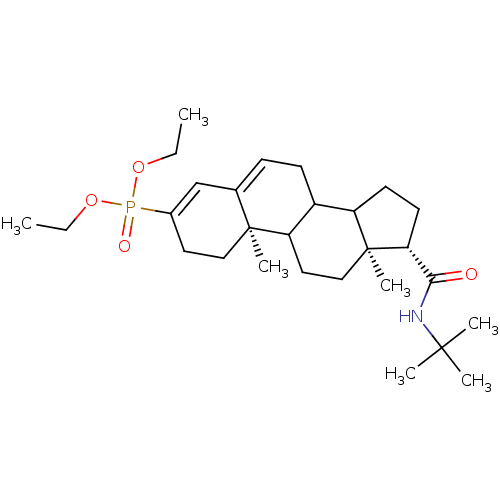

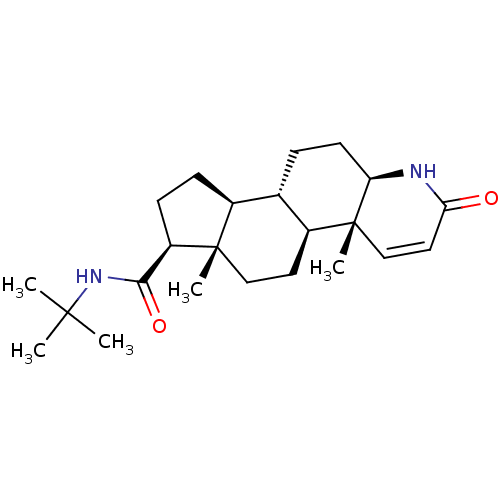
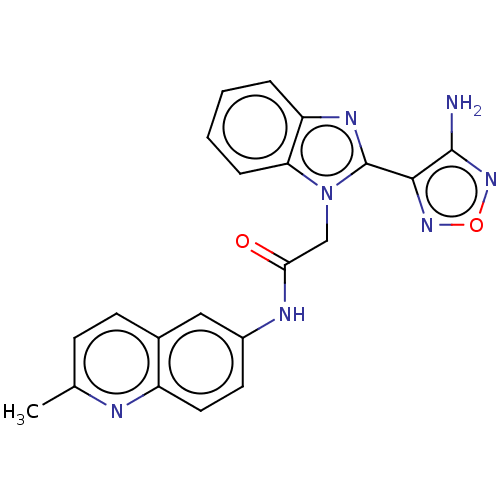
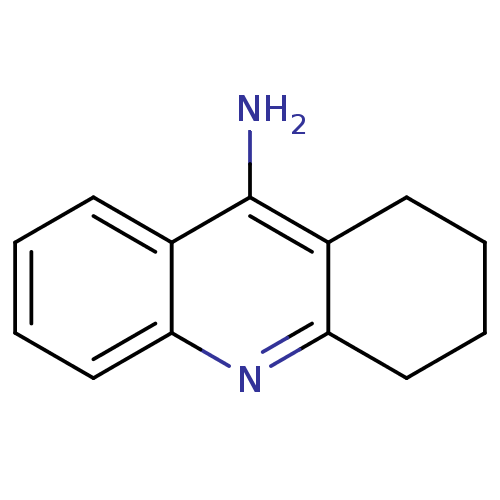
 3D Structure (crystal)
3D Structure (crystal)