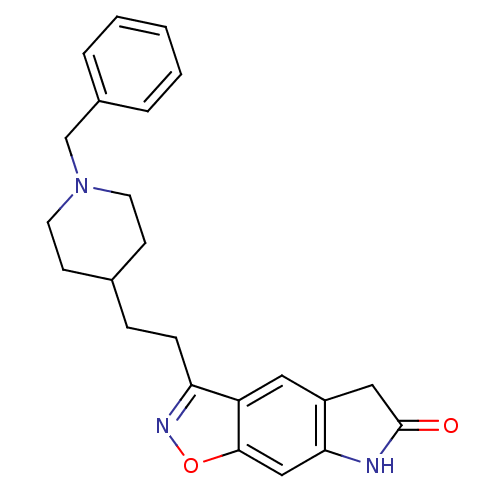
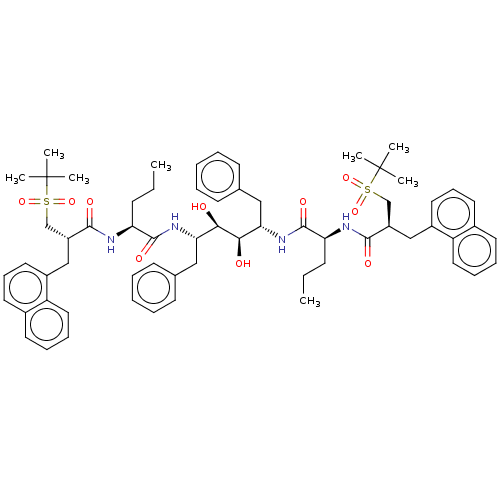
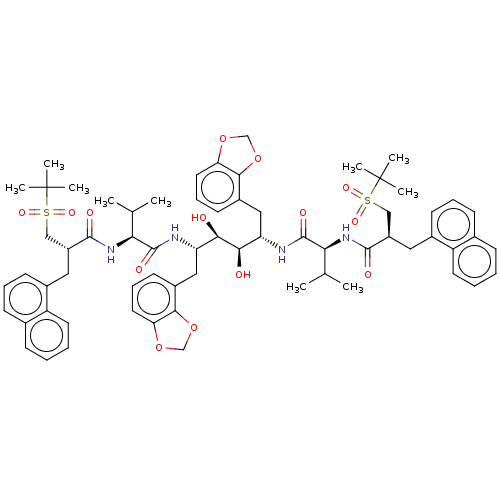
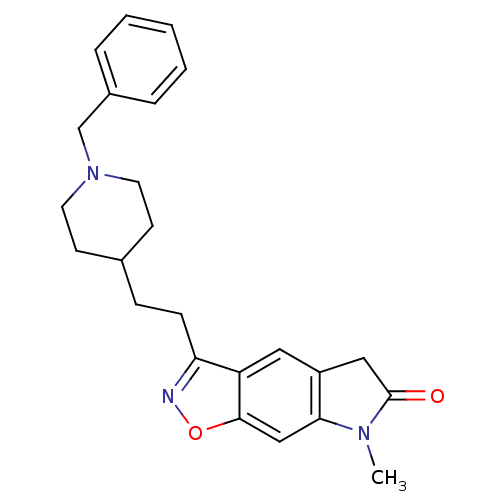
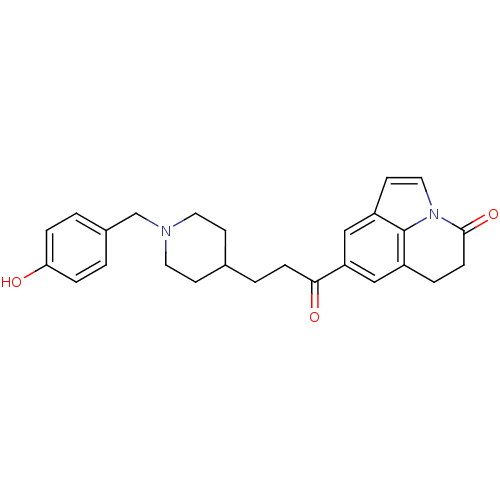
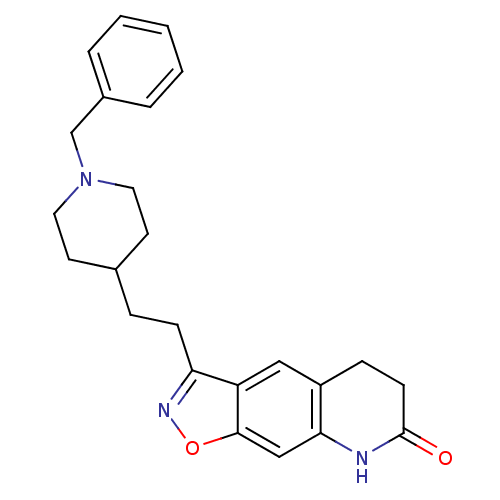
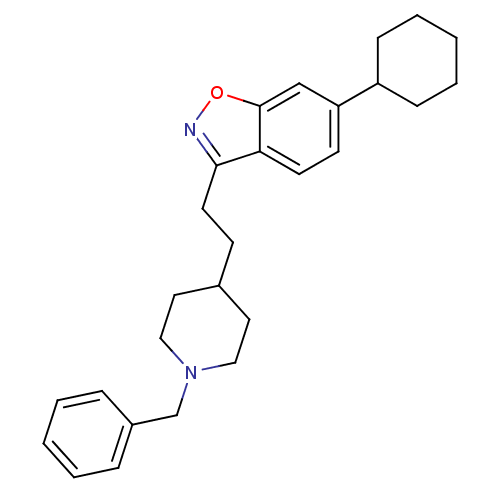
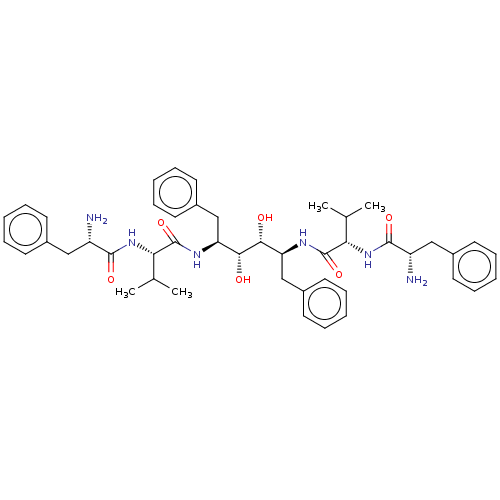
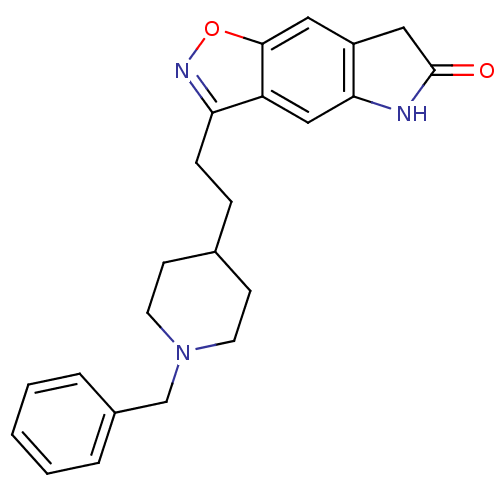
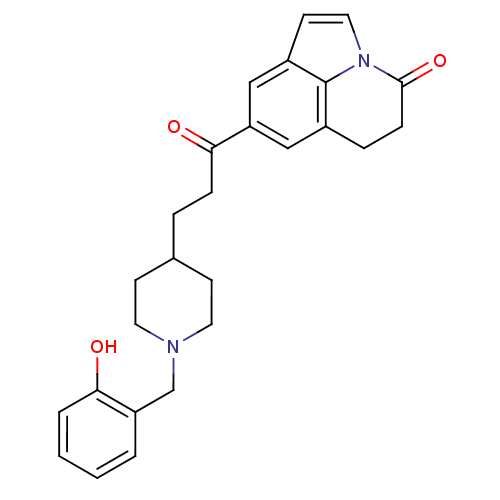
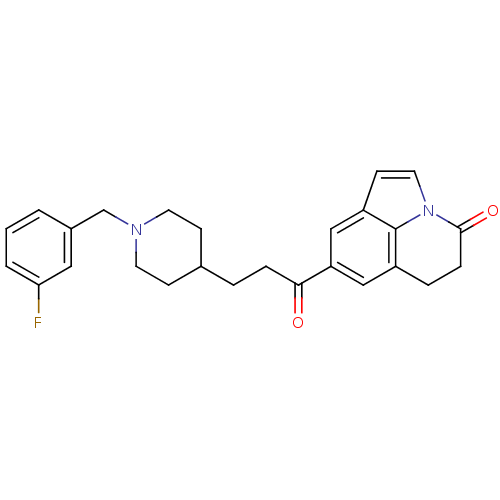
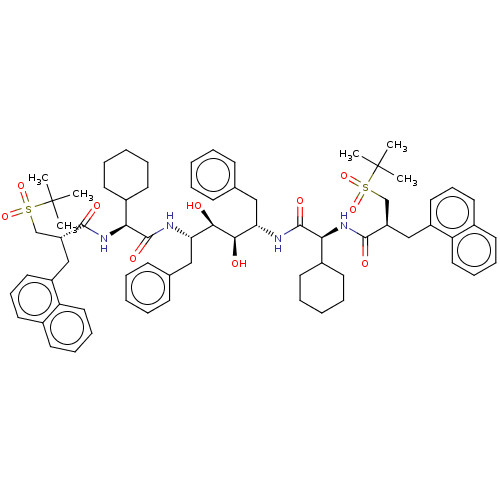
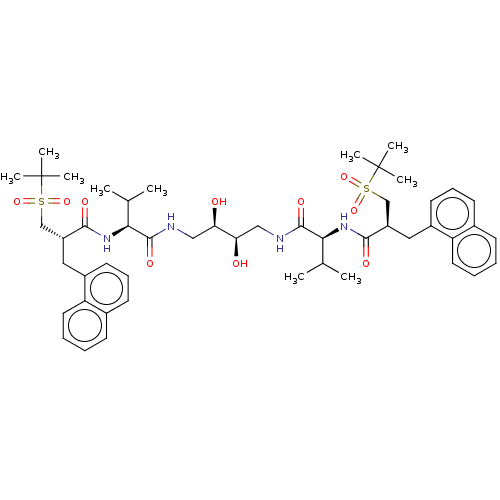
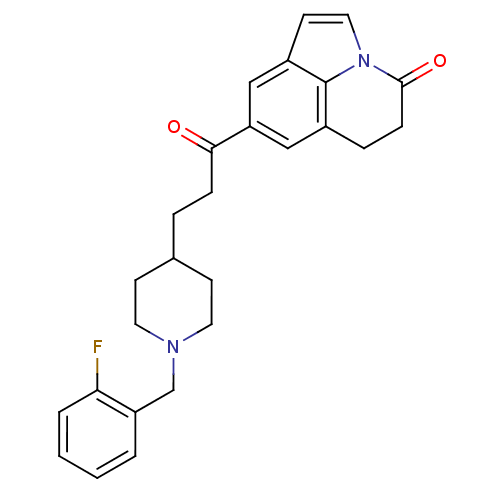
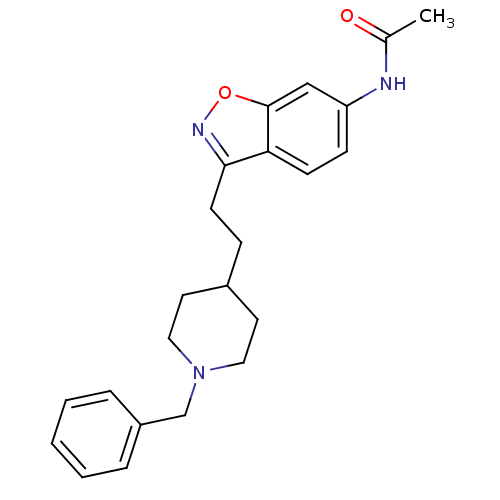
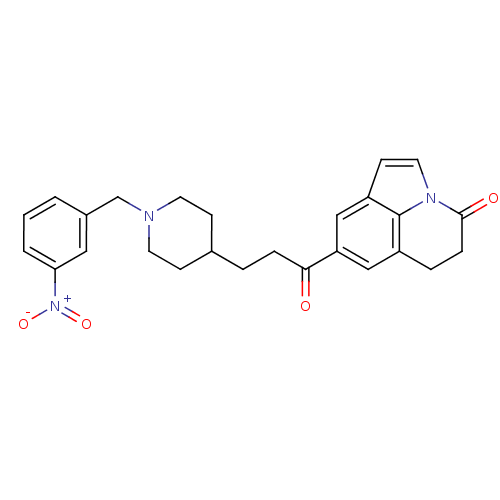
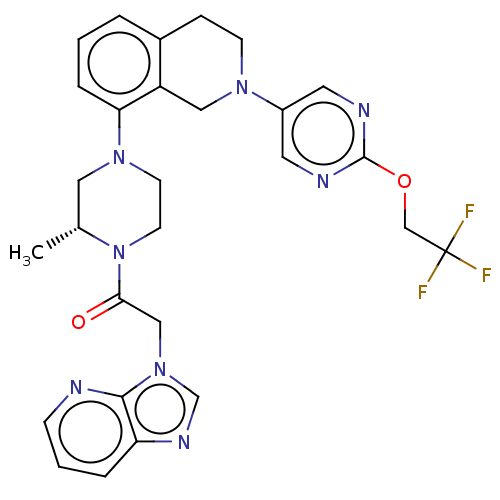
Affinity DataKi: 300nMAssay Description:Binding Affinity to ThrombinMore data for this Ligand-Target Pair
Affinity DataKi: 620nMAssay Description:Binding Affinity to ThrombinMore data for this Ligand-Target Pair
TargetBifunctional purine biosynthesis protein ATIC(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 1.70E+4nMAssay Description:In vitro inhibitory activity against Aminoimidazole carboxamide ribonucleotide transformylaseMore data for this Ligand-Target Pair
TargetTrifunctional purine biosynthetic protein adenosine-3(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 3.50E+4nMAssay Description:In vitro inhibitory activity against Glycinamide ribonucleotide transformylase (GAR Tfase) after 3 min at 250 uMMore data for this Ligand-Target Pair
TargetTrifunctional purine biosynthetic protein adenosine-3(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 4.50E+4nMAssay Description:In vitro inhibitory activity against Glycinamide ribonucleotide transformylase (GAR Tfase)More data for this Ligand-Target Pair
TargetBifunctional purine biosynthesis protein ATIC(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 5.20E+4nMAssay Description:In vitro inhibitory activity against Aminoimidazole carboxamide ribonucleotide transformylaseMore data for this Ligand-Target Pair
TargetTrifunctional purine biosynthetic protein adenosine-3(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:In vitro inhibitory activity against Glycinamide ribonucleotide transformylase (GAR Tfase)More data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Universidade Federal De Lavras
Curated by ChEMBL
Universidade Federal De Lavras
Curated by ChEMBL
Affinity DataIC50: 0.302nMAssay Description:Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activityMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Universidade Federal De Lavras
Curated by ChEMBL
Universidade Federal De Lavras
Curated by ChEMBL
Affinity DataIC50: 0.302nMAssay Description:Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activityMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Universidade Federal De Lavras
Curated by ChEMBL
Universidade Federal De Lavras
Curated by ChEMBL
Affinity DataIC50: 0.302nMAssay Description:Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activityMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Universidade Federal De Lavras
Curated by ChEMBL
Universidade Federal De Lavras
Curated by ChEMBL
Affinity DataIC50: 0.302nMAssay Description:Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activityMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Affinity DataIC50: 0.331nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Universidade Federal De Lavras
Curated by ChEMBL
Universidade Federal De Lavras
Curated by ChEMBL
Affinity DataIC50: 0.339nMAssay Description:Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activityMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Universidade Federal De Lavras
Curated by ChEMBL
Universidade Federal De Lavras
Curated by ChEMBL
Affinity DataIC50: 0.398nMAssay Description:Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activityMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Universidade Federal De Lavras
Curated by ChEMBL
Universidade Federal De Lavras
Curated by ChEMBL
Affinity DataIC50: 0.398nMAssay Description:Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activityMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Affinity DataIC50: 0.479nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Affinity DataIC50: 0.490nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Universidade Federal De Lavras
Curated by ChEMBL
Universidade Federal De Lavras
Curated by ChEMBL
Affinity DataIC50: 0.501nMAssay Description:Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activityMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Affinity DataIC50: 0.575nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Universidade Federal De Lavras
Curated by ChEMBL
Universidade Federal De Lavras
Curated by ChEMBL
Affinity DataIC50: 0.741nMAssay Description:Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activityMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Affinity DataIC50: 0.794nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Universidade Federal De Lavras
Curated by ChEMBL
Universidade Federal De Lavras
Curated by ChEMBL
Affinity DataIC50: 0.794nMAssay Description:Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activityMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Universidade Federal De Lavras
Curated by ChEMBL
Universidade Federal De Lavras
Curated by ChEMBL
Affinity DataIC50: 0.794nMAssay Description:Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activityMore data for this Ligand-Target Pair
Affinity DataIC50: 0.850nMAssay Description:The bioactivity of compounds is tested in a fluorometric imaging plate reader (FLIPR: Molecular Devices) using engineered CHO-K1 cells expressing the...More data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Universidade Federal De Lavras
Curated by ChEMBL
Universidade Federal De Lavras
Curated by ChEMBL
Affinity DataIC50: 0.851nMAssay Description:Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activityMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Universidade Federal De Lavras
Curated by ChEMBL
Universidade Federal De Lavras
Curated by ChEMBL
Affinity DataIC50: 0.891nMAssay Description:Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activityMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Affinity DataIC50: 0.955nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 0.970nMAssay Description:The bioactivity of compounds is tested in a fluorometric imaging plate reader (FLIPR: Molecular Devices) using engineered CHO-K1 cells expressing the...More data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Universidade Federal De Lavras
Curated by ChEMBL
Universidade Federal De Lavras
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activityMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.25nMAssay Description:The bioactivity of compounds is tested in a fluorometric imaging plate reader (FLIPR: Molecular Devices) using engineered CHO-K1 cells expressing the...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Affinity DataIC50: 1.29nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Universidade Federal De Lavras
Curated by ChEMBL
Universidade Federal De Lavras
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activityMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:The bioactivity of compounds is tested in a fluorometric imaging plate reader (FLIPR: Molecular Devices) using engineered CHO-K1 cells expressing the...More data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Universidade Federal De Lavras
Curated by ChEMBL
Universidade Federal De Lavras
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activityMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Universidade Federal De Lavras
Curated by ChEMBL
Universidade Federal De Lavras
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activityMore data for this Ligand-Target Pair
Affinity DataIC50: 1.74nMAssay Description:The bioactivity of compounds is tested in a fluorometric imaging plate reader (FLIPR: Molecular Devices) using engineered CHO-K1 cells expressing the...More data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Universidade Federal De Lavras
Curated by ChEMBL
Universidade Federal De Lavras
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activityMore data for this Ligand-Target Pair
Affinity DataIC50: 2.03nMAssay Description:The bioactivity of compounds is tested in a fluorometric imaging plate reader (FLIPR: Molecular Devices) using engineered CHO-K1 cells expressing the...More data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Universidade Federal De Lavras
Curated by ChEMBL
Universidade Federal De Lavras
Curated by ChEMBL
Affinity DataIC50: 2.20nMAssay Description:Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activityMore data for this Ligand-Target Pair
Affinity DataIC50: 2.26nMAssay Description:The bioactivity of compounds is tested in a fluorometric imaging plate reader (FLIPR: Molecular Devices) using engineered CHO-K1 cells expressing the...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:The bioactivity of compounds is tested in a fluorometric imaging plate reader (FLIPR: Molecular Devices) using engineered CHO-K1 cells expressing the...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Affinity DataIC50: 2.51nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Affinity DataIC50: 2.82nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Universidade Federal Do Rio De Janeiro (Ufrj)
Curated by ChEMBL
Affinity DataIC50: 2.88nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Universidade Federal De Lavras
Curated by ChEMBL
Universidade Federal De Lavras
Curated by ChEMBL
Affinity DataIC50: 2.90nMAssay Description:Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activityMore data for this Ligand-Target Pair
Affinity DataIC50: 2.98nMAssay Description:The bioactivity of compounds is tested in a fluorometric imaging plate reader (FLIPR: Molecular Devices) using engineered CHO-K1 cells expressing the...More data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:The bioactivity of compounds is tested in a fluorometric imaging plate reader (FLIPR: Molecular Devices) using engineered CHO-K1 cells expressing the...More data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Universidade Federal De Lavras
Curated by ChEMBL
Universidade Federal De Lavras
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:Inhibition of HIV1 protease expressed in Escherichia coli K12 assessed as inhibition of enzyme activityMore data for this Ligand-Target Pair
Affinity DataIC50: 3.39nMAssay Description:The bioactivity of compounds is tested in a fluorometric imaging plate reader (FLIPR: Molecular Devices) using engineered CHO-K1 cells expressing the...More data for this Ligand-Target Pair



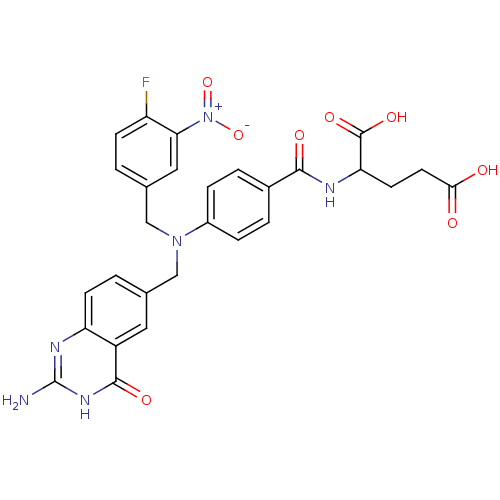
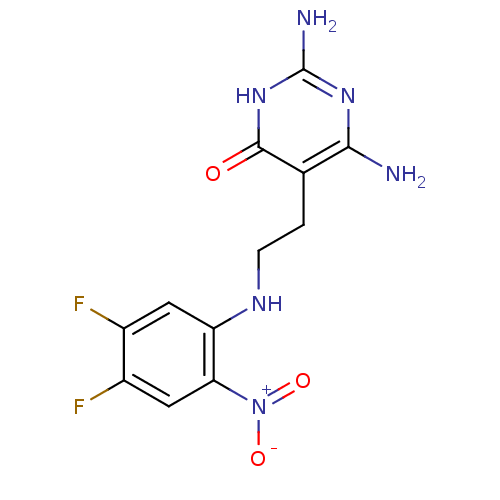
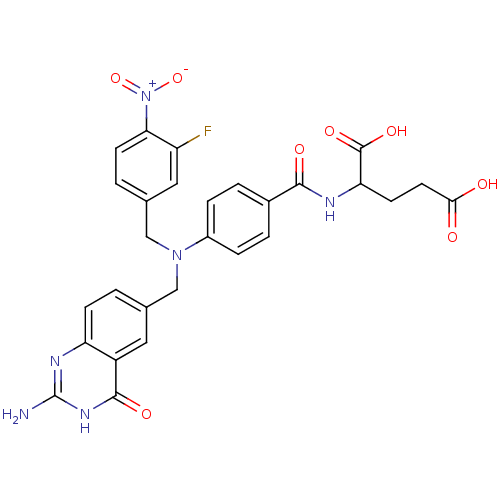



 3D Structure (crystal)
3D Structure (crystal)