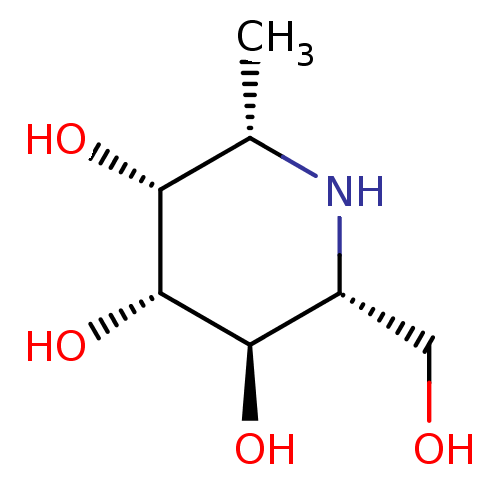
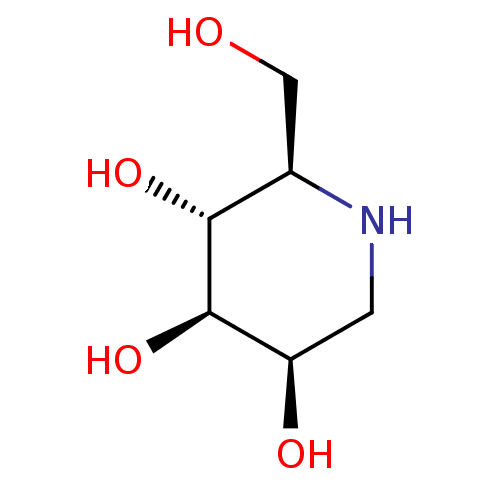
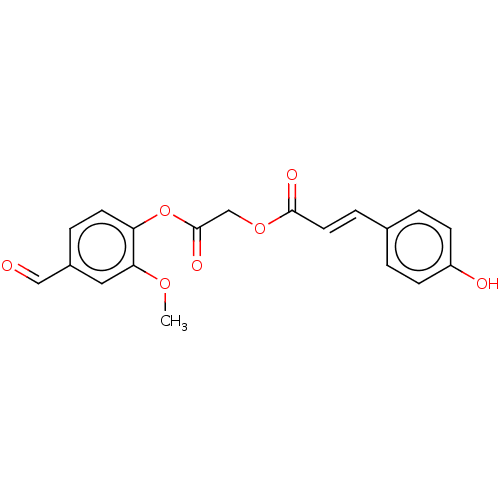
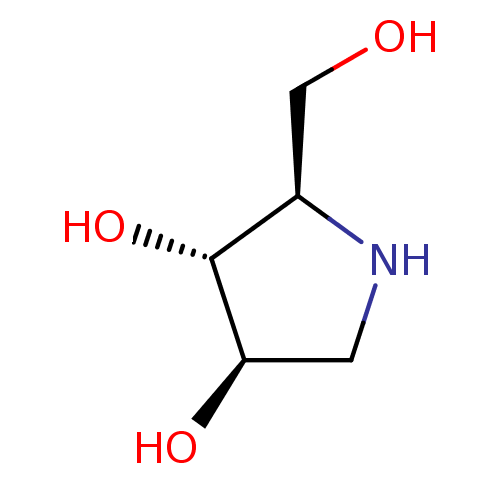
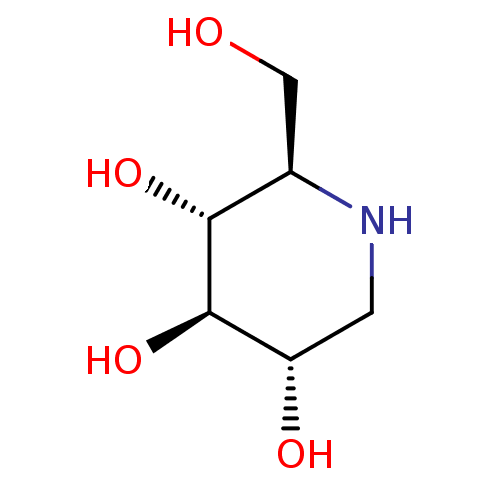
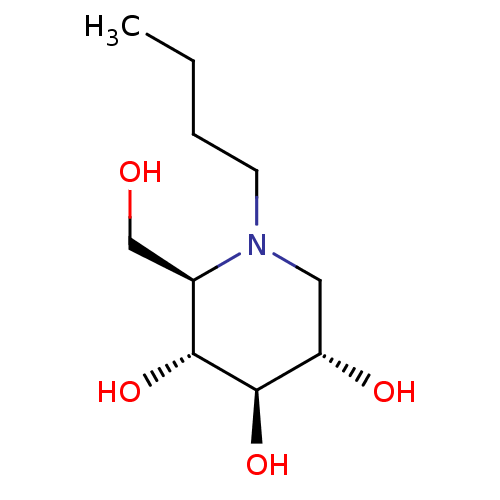
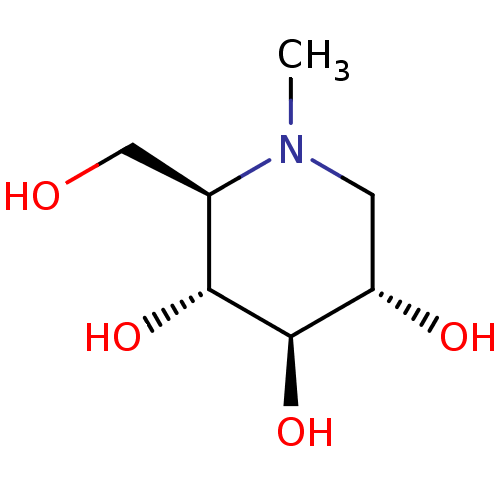
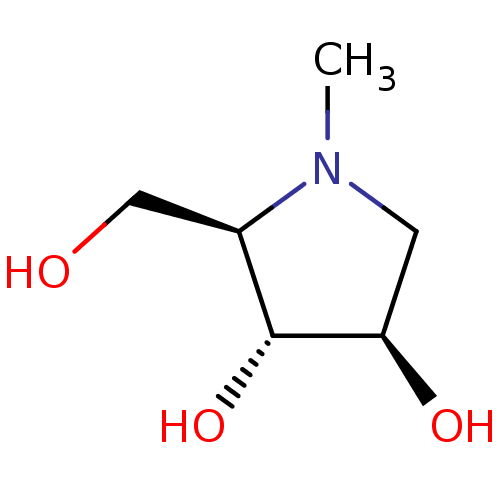
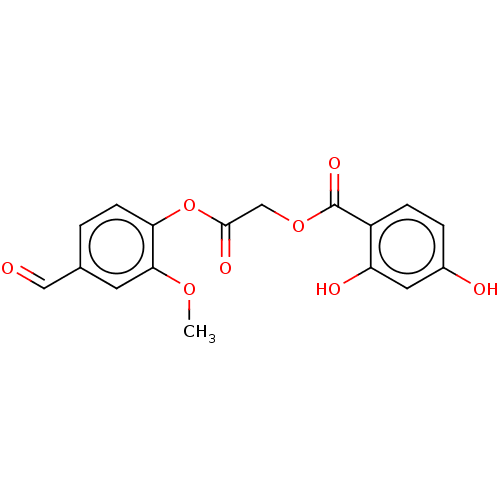
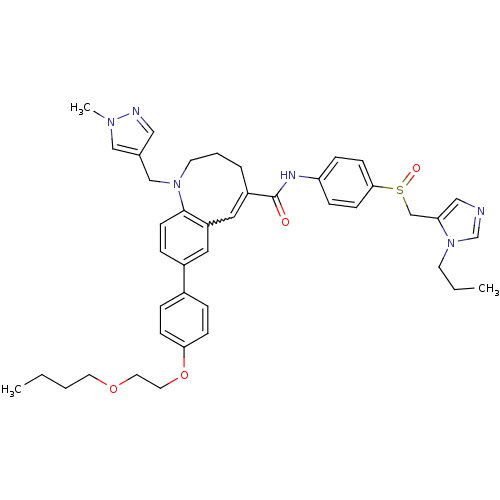
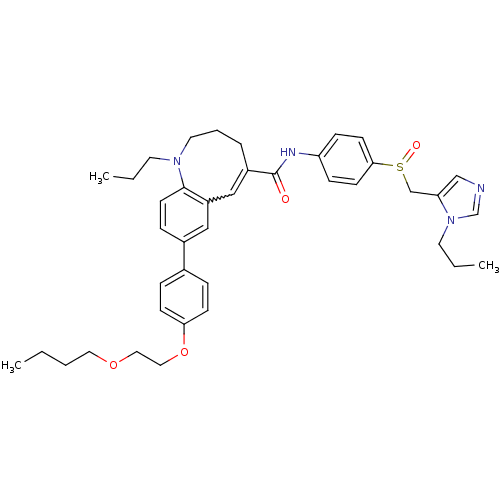
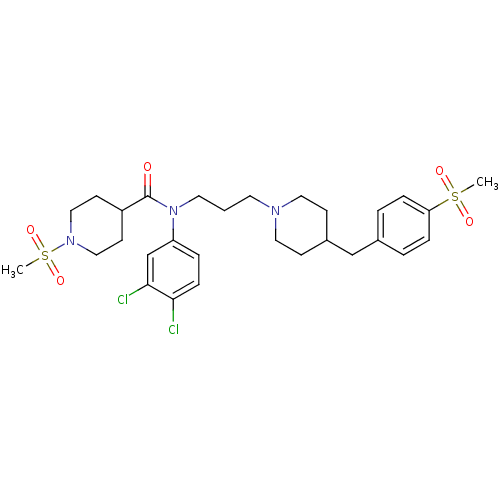
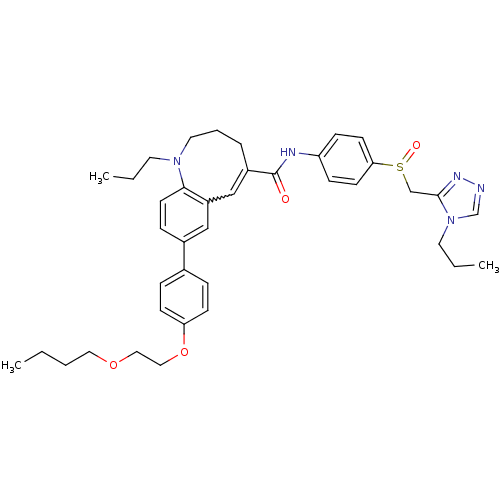
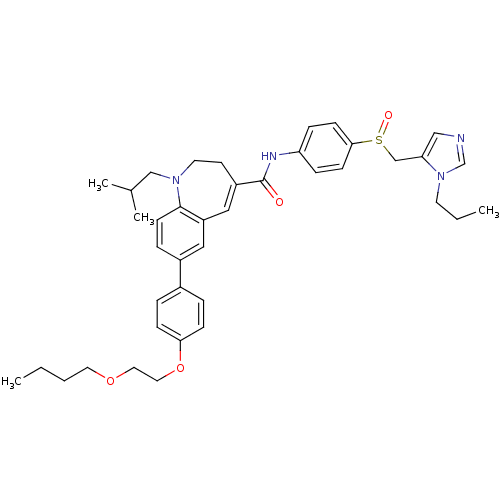
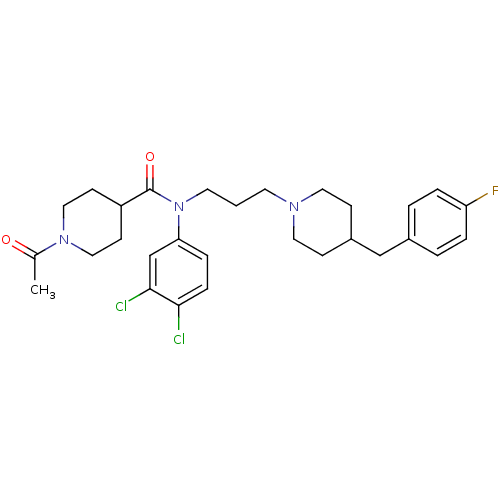
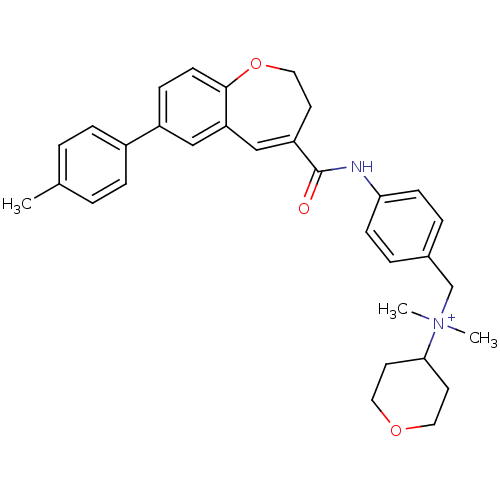
Affinity DataKi: 24nMAssay Description:Inhibition of HIV1 Reverse transcriptase P119S/T165A mutantMore data for this Ligand-Target Pair
Affinity DataKi: 54nMAssay Description:Inhibition of wild-type HIV1 Reverse transcriptaseMore data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Inhibition of HIV1 Reverse transcriptase T165A mutantMore data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Inhibitory activity measured against alpha-L-fucosidase of bovine epididymis by colorimetric assay using the D-glucose oxidase-peroxidase methodMore data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Inhibitory activity measured against alpha-L-fucosidase of bovine epididymis by colorimetric assay using the D-glucose oxidase-peroxidase methodMore data for this Ligand-Target Pair
Affinity DataKi: 110nMAssay Description:Inhibition of HIV1 Reverse transcriptase P119S mutantMore data for this Ligand-Target Pair
Affinity DataKi: 210nMAssay Description:Inhibition of HIV1 Reverse transcriptase M184V mutantMore data for this Ligand-Target Pair
Affinity DataKi: 210nMAssay Description:Inhibition of HIV1 Reverse transcriptase T165A mutantMore data for this Ligand-Target Pair
Affinity DataKi: 370nMAssay Description:Inhibition of HIV1 Reverse transcriptase P119S/T165A mutantMore data for this Ligand-Target Pair
Affinity DataKi: 450nMAssay Description:Compound was tested for binding affinity against alpha-glucosidaseMore data for this Ligand-Target Pair
Affinity DataKi: 480nMAssay Description:Inhibition of HIV1 Reverse transcriptase P119S/M184V mutantMore data for this Ligand-Target Pair
Affinity DataKi: 630nMAssay Description:Inhibition of wild-type HIV1 Reverse transcriptaseMore data for this Ligand-Target Pair
Affinity DataKi: 640nMAssay Description:Inhibition of HIV1 Reverse transcriptase P119S/M184V mutantMore data for this Ligand-Target Pair
Affinity DataKi: 640nMAssay Description:Inhibition of HIV1 Reverse transcriptase P119S mutantMore data for this Ligand-Target Pair
Affinity DataKi: 730nMAssay Description:Inhibition of HIV1 Reverse transcriptase P119S/T165A/M184V mutantMore data for this Ligand-Target Pair
Affinity DataKi: 790nMAssay Description:Inhibition of HIV1 Reverse transcriptase M184V mutantMore data for this Ligand-Target Pair
Affinity DataKi: 1.05E+3nMAssay Description:Inhibition of HIV1 Reverse transcriptase P119S/T165A/M184V mutantMore data for this Ligand-Target Pair
Affinity DataKi: 4.70E+3nMAssay Description:Inhibitory activity measured against alpha-L-fucosidase of bovine epididymis by colorimetric assay using the D-glucose oxidase-peroxidase methodMore data for this Ligand-Target Pair
Affinity DataKi: 6.20E+3nMAssay Description:Compound was tested for binding affinity against alpha-glucosidaseMore data for this Ligand-Target Pair
Affinity DataKi: 6.90E+3nMAssay Description:Compound was tested for binding affinity against alpha-glucosidaseMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Kongju National University
Curated by ChEMBL
Kongju National University
Curated by ChEMBL
Affinity DataKi: 1.30E+4nMAssay Description:Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-inhibitor dissociation constant preincubated for 10 mins f...More data for this Ligand-Target Pair
Affinity DataKi: 3.50E+4nMAssay Description:Competitive Inhibitory activity against Golgi Alpha-mannosidase IIMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Kongju National University
Curated by ChEMBL
Kongju National University
Curated by ChEMBL
Affinity DataKi: 4.10E+4nMAssay Description:Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-inhibitor dissociation constant preincubated for 10 mins f...More data for this Ligand-Target Pair
Affinity DataKi: <5.00E+4nMAssay Description:Competitive Inhibitory activity against Golgi Alpha-mannosidase IIMore data for this Ligand-Target Pair
Affinity DataKi: <5.00E+4nMAssay Description:Competitive Inhibitory activity against Golgi Alpha-mannosidase IIMore data for this Ligand-Target Pair
Affinity DataKi: <5.00E+4nMAssay Description:Competitive Inhibitory activity against Golgi Alpha-mannosidase IIMore data for this Ligand-Target Pair
Affinity DataKi: 5.10E+4nMAssay Description:Competitive Inhibitory activity against Golgi Alpha-mannosidase IIMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Kongju National University
Curated by ChEMBL
Kongju National University
Curated by ChEMBL
Affinity DataKi: 5.30E+4nMAssay Description:Non-competitive inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-substrate-inhibitor dissociation constant preincubated...More data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Kongju National University
Curated by ChEMBL
Kongju National University
Curated by ChEMBL
Affinity DataKi: 6.00E+4nMAssay Description:Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-inhibitor dissociation constant preincubated for 10 mins f...More data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Kongju National University
Curated by ChEMBL
Kongju National University
Curated by ChEMBL
Affinity DataKi: 6.50E+4nMAssay Description:Competitive inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-inhibitor dissociation constant preincubated for 10 mins f...More data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Kongju National University
Curated by ChEMBL
Kongju National University
Curated by ChEMBL
Affinity DataKi: 1.00E+5nMAssay Description:Non-competitive inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-substrate-inhibitor dissociation constant preincubated...More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+5nMAssay Description:Competitive Inhibitory activity against Golgi Alpha-mannosidase IIMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Kongju National University
Curated by ChEMBL
Kongju National University
Curated by ChEMBL
Affinity DataKi: 2.05E+5nMAssay Description:Non-competitive inhibition of mushroom tyrosinase using L-DOPA as substrate assessed as enzyme-substrate-inhibitor dissociation constant preincubated...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5More data for this Ligand-Target Pair
Affinity DataIC50: 0.190nMAssay Description:Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.210nMAssay Description:Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5More data for this Ligand-Target Pair
Affinity DataIC50: 0.240nMAssay Description:Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5More data for this Ligand-Target Pair
Affinity DataIC50: 0.420nMAssay Description:Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5More data for this Ligand-Target Pair
Affinity DataIC50: 0.480nMAssay Description:Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5More data for this Ligand-Target Pair
Affinity DataIC50: 0.950nMAssay Description:Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Inhibition of [125I]RANTES binding to human CCR5 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTESMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTESMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Displacement of [125I]RANTES from CCR5 expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of replication of R5 HIV1 Ba-L in MOLT4/CCR5 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of membrane fusion between HIV1 JR-FL Env-expressing COS7 cells and MOLT4/CCR5More data for this Ligand-Target Pair



 3D Structure (crystal)
3D Structure (crystal)