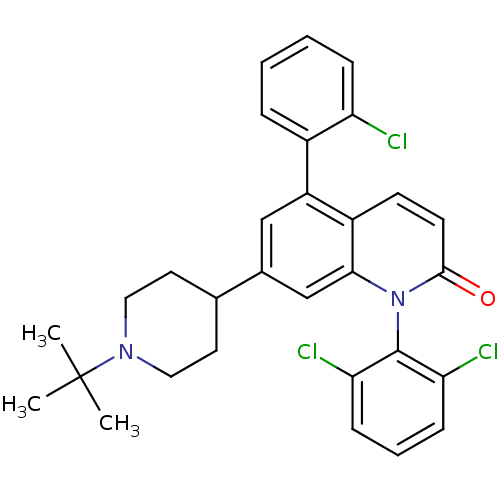
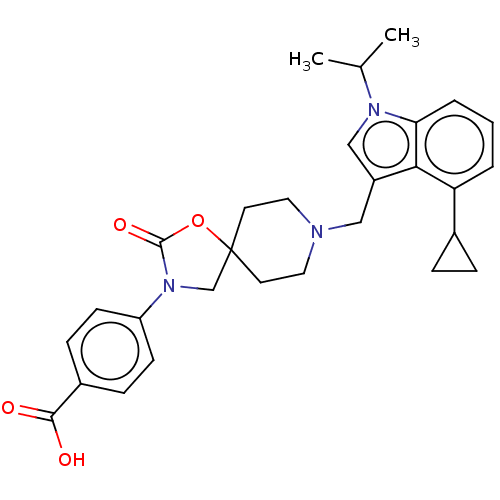
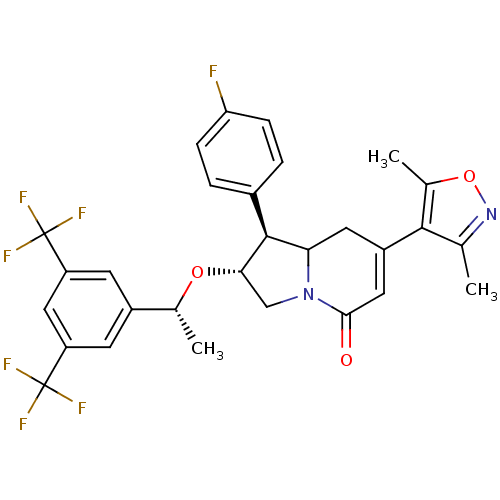
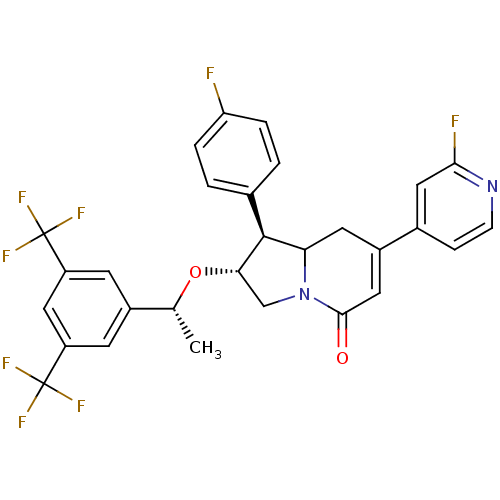
Affinity DataKi: 0.200nM ΔG°: -51.5kJ/mole IC50: 0.200nM EC50: 0.200nMpH: 7.5 T: 2°CAssay Description:Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nM ΔG°: -48.9kJ/mole IC50: 1.10nM EC50: 5nMpH: 7.5 T: 2°CAssay Description:Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nM ΔG°: -48.6kJ/mole IC50: 0.200nM EC50: 0.200nMpH: 7.5 T: 2°CAssay Description:Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the...More data for this Ligand-Target Pair
Affinity DataKi: 1.10nM ΔG°: -47.5kJ/mole IC50: 0.200nM EC50: 0.100nMpH: 7.5 T: 2°CAssay Description:Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the...More data for this Ligand-Target Pair
Affinity DataKi: 1.20nM ΔG°: -47.3kJ/mole IC50: 1.60nM EC50: 1.30nMpH: 7.5 T: 2°CAssay Description:Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the...More data for this Ligand-Target Pair
Affinity DataKi: 1.40nM ΔG°: -47.0kJ/mole IC50: 0.400nM EC50: 0.300nMpH: 7.5 T: 2°CAssay Description:Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the...More data for this Ligand-Target Pair
Affinity DataKi: 2.10nM ΔG°: -46.0kJ/mole IC50: 1.40nM EC50: 0.200nMpH: 7.5 T: 2°CAssay Description:Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the...More data for this Ligand-Target Pair
Affinity DataKi: 4.40nM ΔG°: -44.3kJ/mole IC50: 6.10nM EC50: 0.5nMpH: 7.5 T: 2°CAssay Description:Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the...More data for this Ligand-Target Pair
Affinity DataKi: 7.20nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 38nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 42nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 48nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 57nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 110nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 150nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 190nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 290nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 290nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 346nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 410nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 670nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 790nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 980nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 1.40E+3nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 1.53E+3nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 1.78E+3nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 1.90E+3nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 2.29E+3nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0130nMAssay Description:Displacement of [125I]substance P from human NK1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0410nMAssay Description:Displacement of [125I]substance P from human NK1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0600nMAssay Description:Displacement of [125I]substance P from human NK1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0600nMAssay Description:Displacement of [125I]substance P from human NK1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0660nMAssay Description:Displacement of [125I]substance P from human NK1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0690nMAssay Description:TrkA functional activity was measured using a DiscoverX PathHunter assay. In this assay, U2OS cells express the human TrkA receptor as a fusion with ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0830nMAssay Description:TrkA functional activity was measured using a DiscoverX PathHunter assay. In this assay, U2OS cells express the human TrkA receptor as a fusion with ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0900nMAssay Description:Displacement of [125I]substance P from human NK1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of P38 alpha MAPKMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Kallikrein determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaC12, and 0.1% PEG 8000 (polyethylene glycol; Fishe...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Displacement of [125I]substance P from human NK1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.110nMAssay Description:TrkA functional activity was measured using a DiscoverX PathHunter assay. In this assay, U2OS cells express the human TrkA receptor as a fusion with ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.110nMAssay Description:Displacement of [125I]substance P from human NK1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.110nMAssay Description:Displacement of [125I]substance P from human NK1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.110nMAssay Description:Displacement of [125I]substance P from human NK1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.110nMAssay Description:Displacement of [125I]substance P from human NK1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.129nMpH: 7.8Assay Description:SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et...More data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMAssay Description:Displacement of [125I]substance P from human NK1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.140nMAssay Description:Displacement of [125I]substance P from human NK1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair

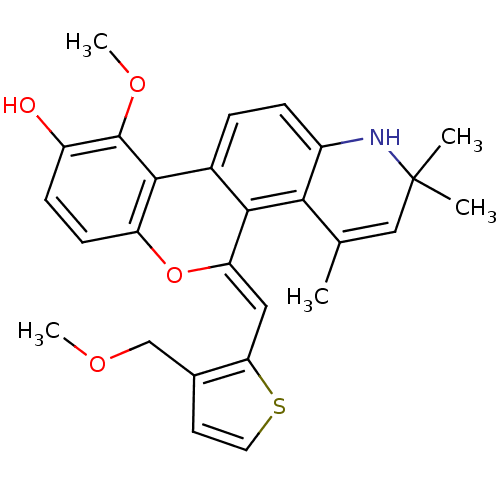
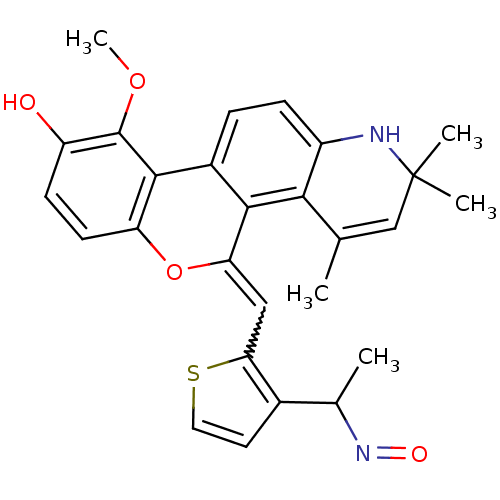
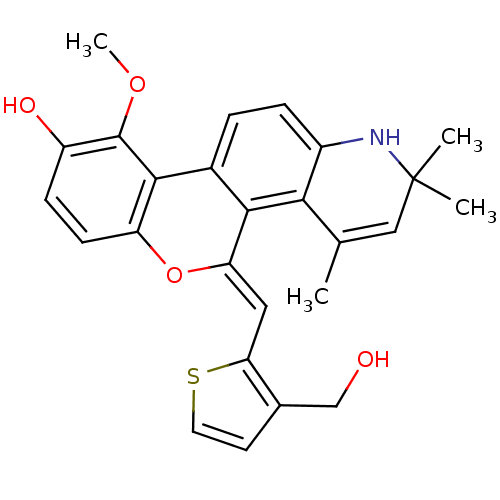
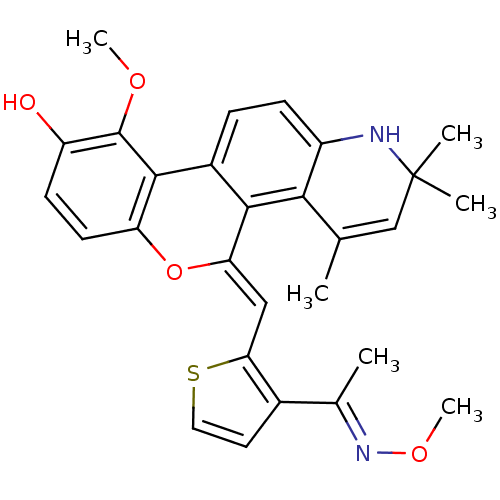

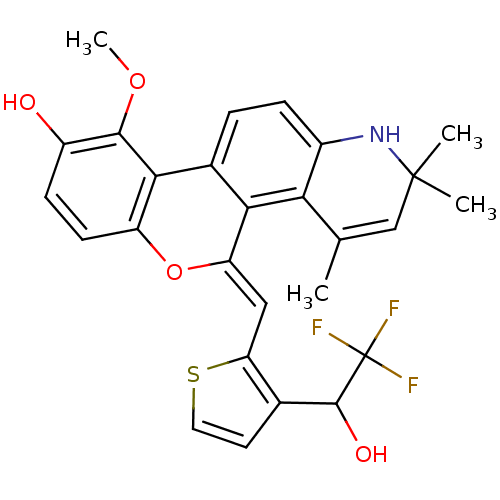
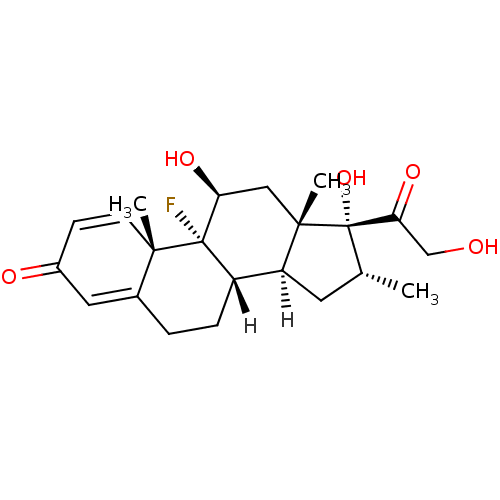
 3D Structure (crystal)
3D Structure (crystal)