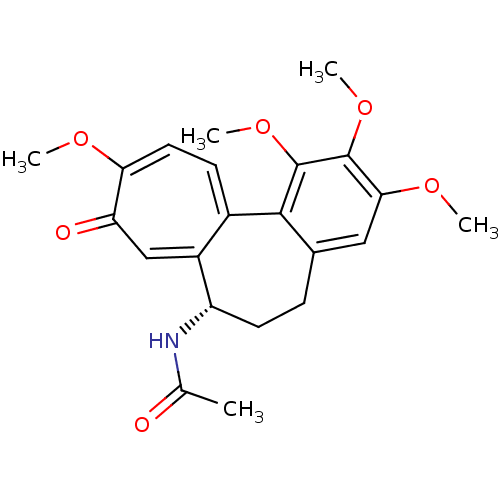
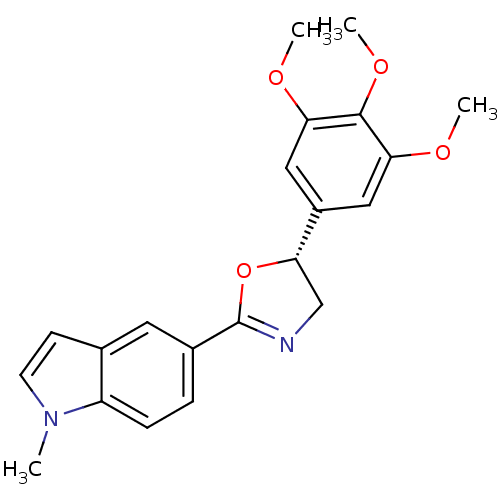
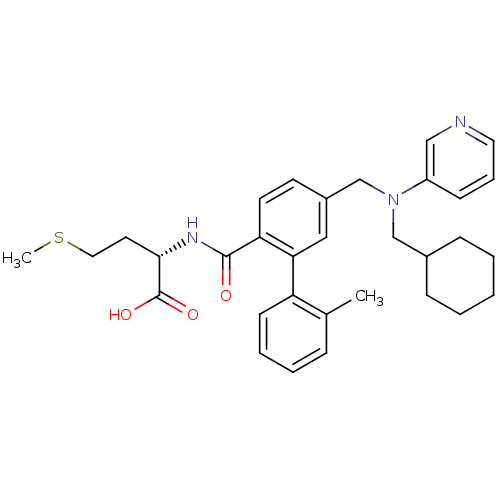
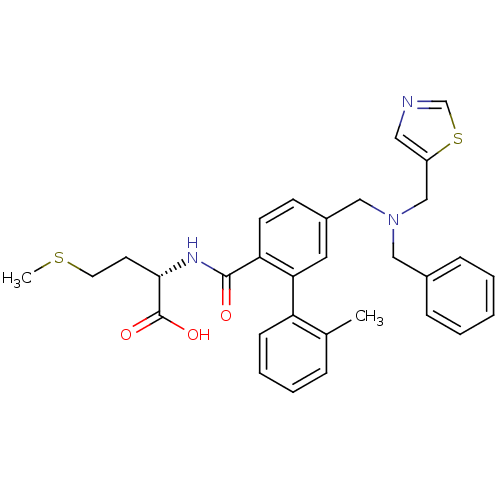
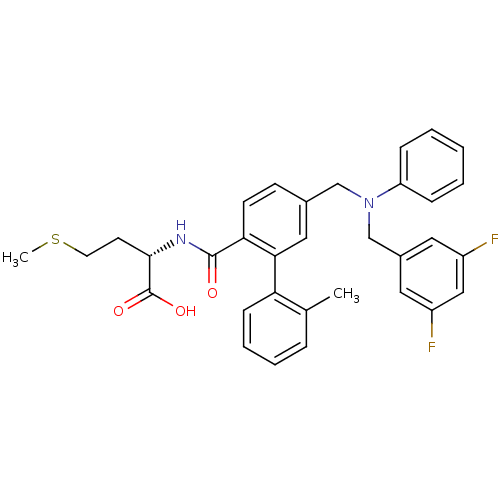
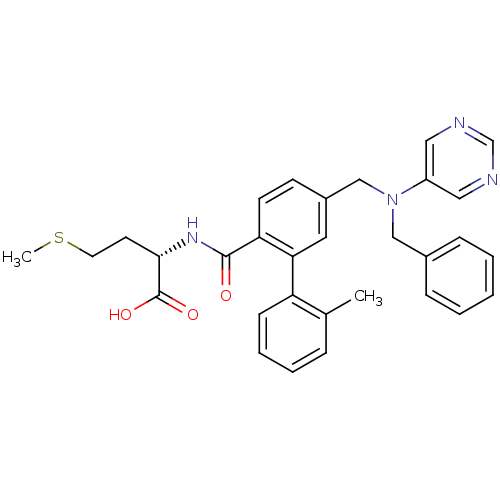
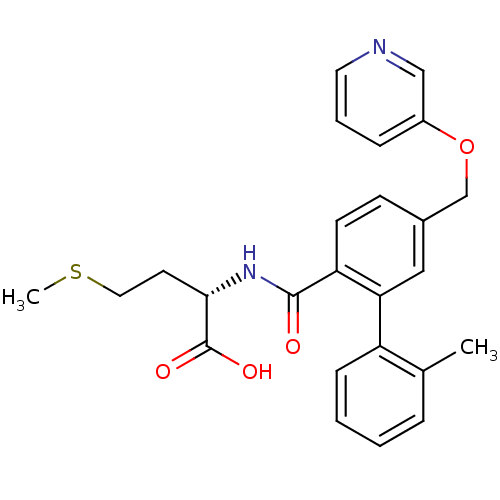
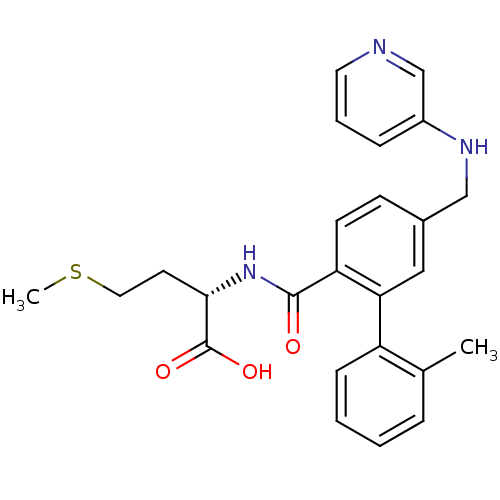
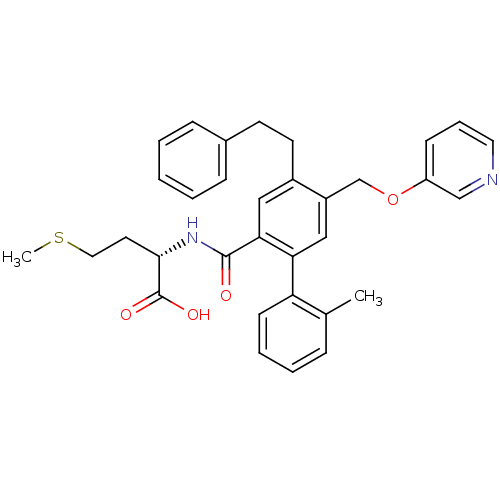
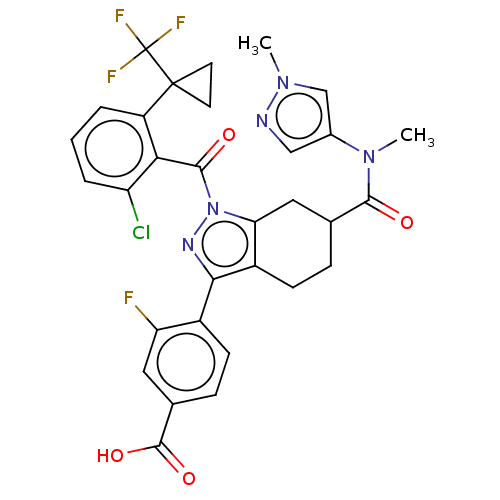
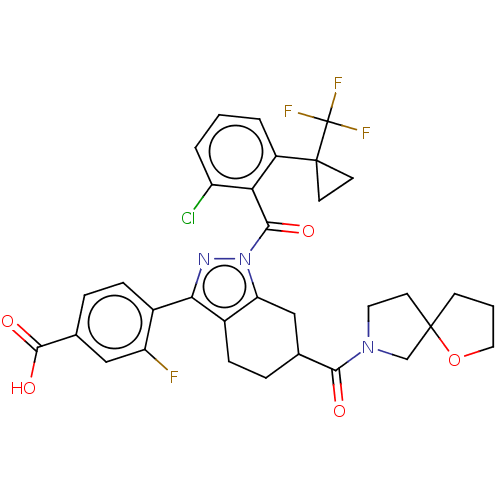
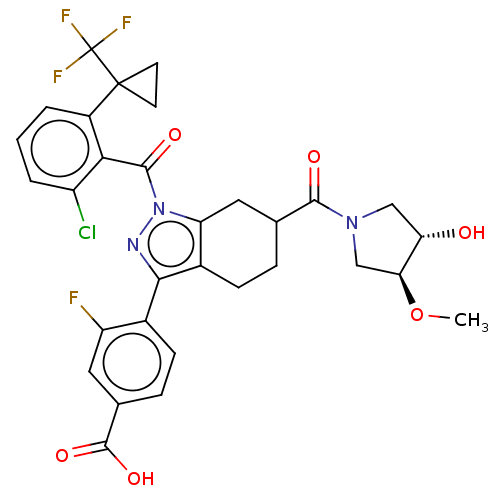
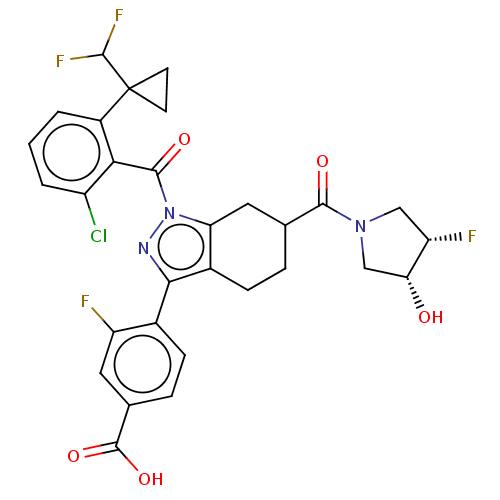
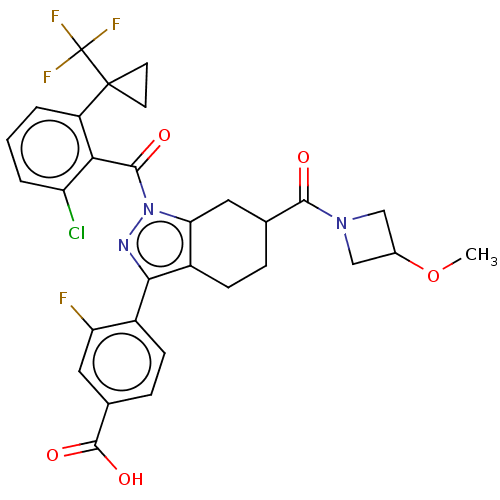
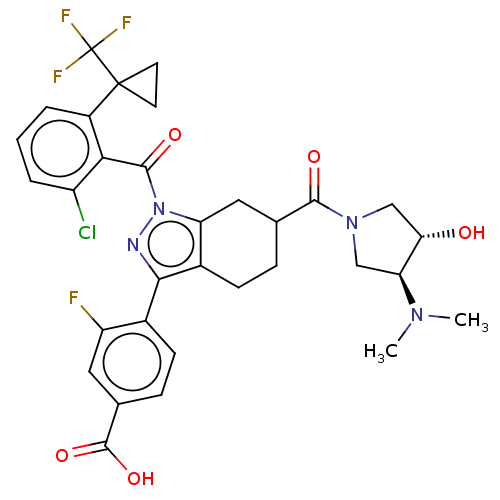
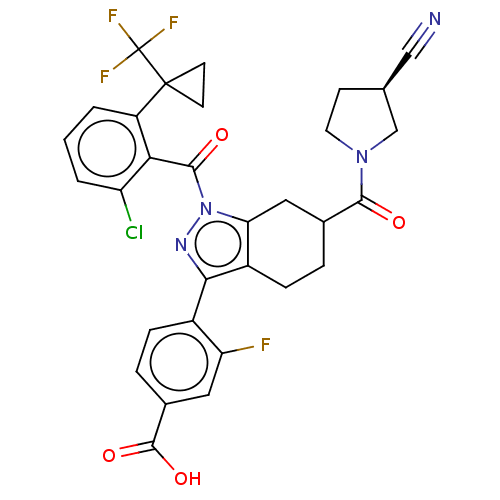
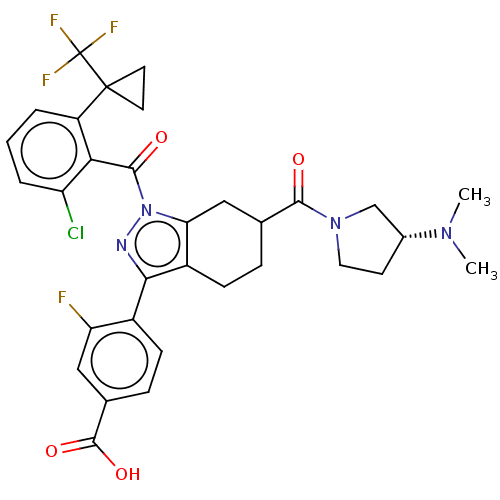
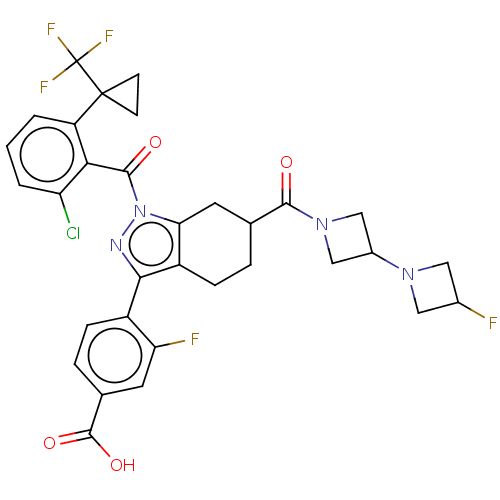
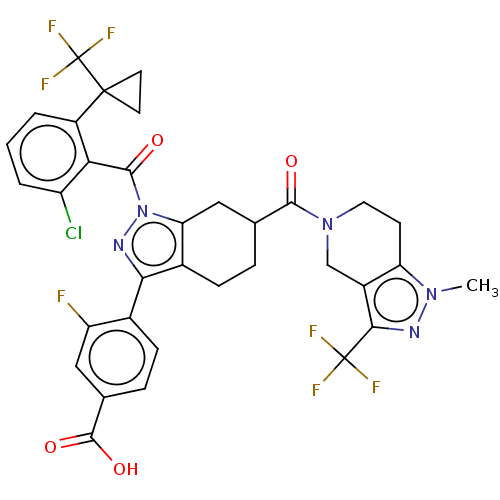
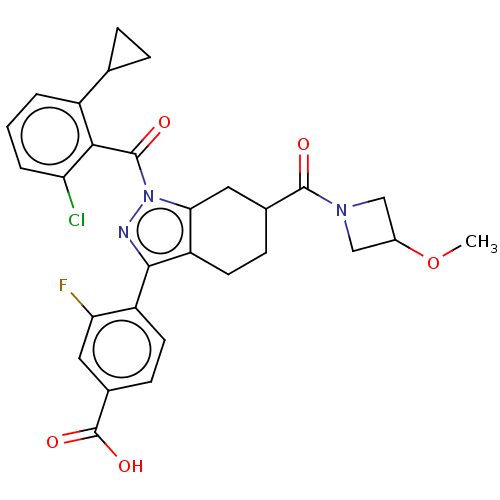
Affinity DataKi: 180nMAssay Description:Binding constant at colchicine site of bovine brain tubulinMore data for this Ligand-Target Pair
Affinity DataKi: 630nMAssay Description:Binding constant at colchicine site of bovine brain tubulinMore data for this Ligand-Target Pair
Affinity DataKi: 780nMAssay Description:Binding constant at colchicine site of bovine brain tubulinMore data for this Ligand-Target Pair
Affinity DataKi: 3.69E+3nMAssay Description:Binding constant at colchicine site of bovine brain tubulinMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding constant at colchicine site of bovine brain tubulinMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:In vitro inhibition of farnesyltransferase purified from bovine brain using scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:In vitro inhibition of farnesyltransferase purified from bovine brain using scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:In vitro inhibition of farnesyltransferase purified from bovine brain using scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:In vitro inhibition of farnesyltransferase purified from bovine brain using scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:In vitro inhibition of farnesyltransferase purified from bovine brain using scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:In vitro inhibition of farnesyltransferase purified from bovine brain using scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:In vitro inhibition of farnesyltransferase purified from bovine brain using scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:In vitro inhibition of farnesyltransferase purified from bovine brain using scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:In vitro inhibition of farnesyltransferase purified from bovine brain using scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:In vitro inhibition of farnesyltransferase purified from bovine brain using scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:In vitro inhibition of farnesyltransferase purified from bovine brain using scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic(Homo sapiens (Human))
Forma Therapeutics
Curated by ChEMBL
Forma Therapeutics
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human Myc-DDK-tagged IDH1 R132H mutant expressed in human U87MG cells assessed as reduction in 2-HG levels after 24 hrs by RapidFire hi...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)