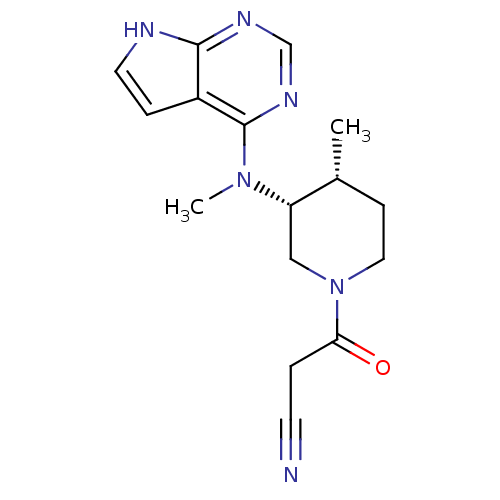
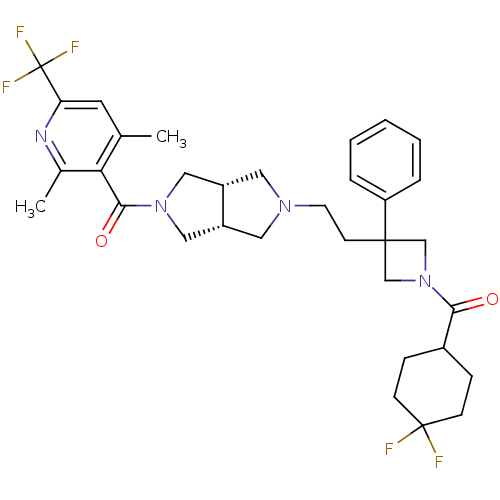
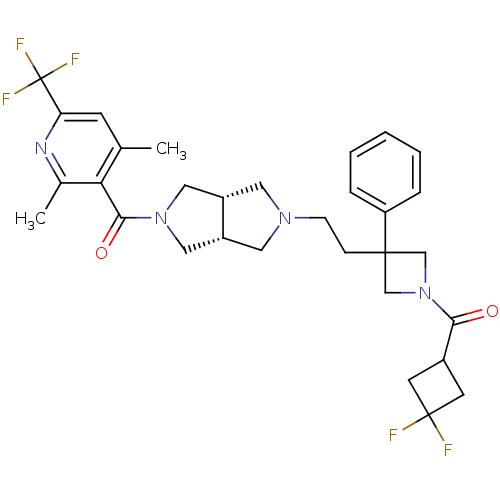
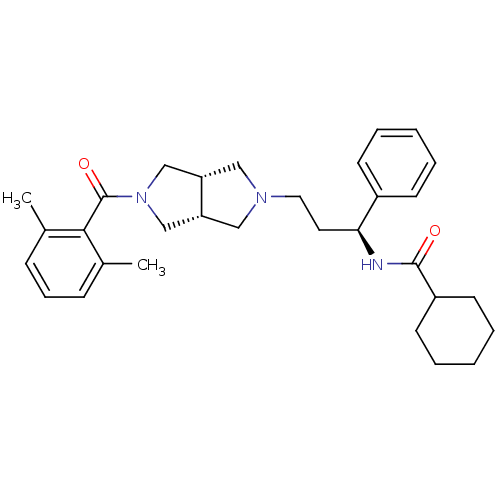
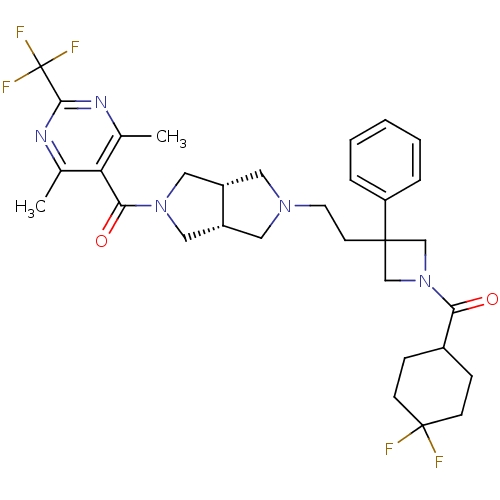
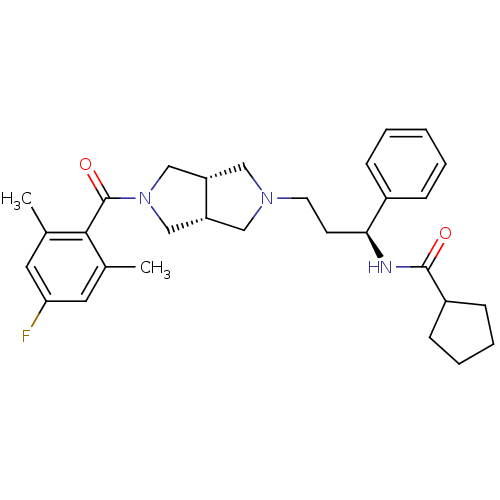
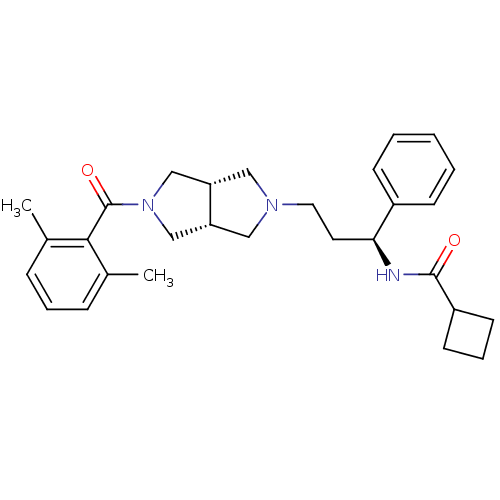
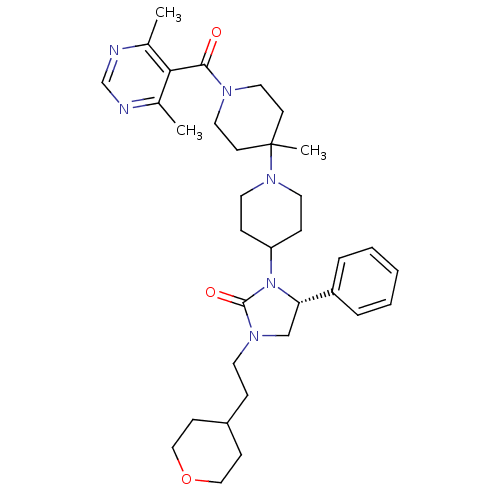
Affinity DataKi: 7.60nMAssay Description:Binding affinity for Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Apparent Ki for rat Lanosterol 14-alpha demethylaseMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Binding affinity for Cytochrome P450 19A1More data for this Ligand-Target Pair
TargetCytochrome P450 11B1, mitochondrial(Homo sapiens (Human))
Syntex Discovery Research
Curated by ChEMBL
Syntex Discovery Research
Curated by ChEMBL
Affinity DataKi: 15nMAssay Description:Binding affinity for corticoid 11-beta-hydroxylaseMore data for this Ligand-Target Pair
TargetLanosterol 14-alpha demethylase(Homo sapiens (Human))
Syntex Discovery Research
Curated by ChEMBL
Syntex Discovery Research
Curated by ChEMBL
Affinity DataKi: 25nMAssay Description:Inhibition of lanosterol 14-alpha-demethylase in hamster hepatic microsomesMore data for this Ligand-Target Pair
Affinity DataKi: 28nMAssay Description:Binding affinity for progesterone 6-beta-hydroxylase of hepatic microsomesMore data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:Binding affinity for progesterone 6-beta-hydroxylase of hepatic microsomesMore data for this Ligand-Target Pair
TargetCytochrome P450 11B1, mitochondrial(Homo sapiens (Human))
Syntex Discovery Research
Curated by ChEMBL
Syntex Discovery Research
Curated by ChEMBL
Affinity DataKi: 35nMAssay Description:Binding affinity for corticoid 11-beta-hydroxylaseMore data for this Ligand-Target Pair
Affinity DataKi: 37nMAssay Description:Inhibition of lanosterol 14-alpha-demethylase of rat hepatic microsomesMore data for this Ligand-Target Pair
TargetLanosterol 14-alpha demethylase(Homo sapiens (Human))
Syntex Discovery Research
Curated by ChEMBL
Syntex Discovery Research
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Inhibition of lanosterol 14-alpha-demethylase in hamster hepatic microsomesMore data for this Ligand-Target Pair
TargetLanosterol 14-alpha demethylase(Homo sapiens (Human))
Syntex Discovery Research
Curated by ChEMBL
Syntex Discovery Research
Curated by ChEMBL
Affinity DataKi: 40.4nMAssay Description:Inhibition of lanosterol 14-alpha-demethylase in hamster hepatic microsomesMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Syntex Discovery Research
Curated by ChEMBL
Syntex Discovery Research
Curated by ChEMBL
Affinity DataKi: 54nMAssay Description:Binding affinity for progesterone 17alpha,20-lyaseMore data for this Ligand-Target Pair
TargetLanosterol 14-alpha demethylase(Homo sapiens (Human))
Syntex Discovery Research
Curated by ChEMBL
Syntex Discovery Research
Curated by ChEMBL
Affinity DataKi: 64nMAssay Description:Inhibition of lanosterol 14-alpha-demethylase in hamster hepatic microsomesMore data for this Ligand-Target Pair
Affinity DataKi: 65nMAssay Description:Inhibition of lanosterol 14-alpha-demethylase of rat hepatic microsomesMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Syntex Discovery Research
Curated by ChEMBL
Syntex Discovery Research
Curated by ChEMBL
Affinity DataKi: 109nMAssay Description:Binding affinity for cholesterol 17-alpha-hydroxylaseMore data for this Ligand-Target Pair
Affinity DataKi: 117nMAssay Description:Apparent Ki for rat Lanosterol 14-alpha demethylaseMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Syntex Discovery Research
Curated by ChEMBL
Syntex Discovery Research
Curated by ChEMBL
Affinity DataKi: 447nMAssay Description:Binding affinity for progesterone 17-alpha,20-lyaseMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Syntex Discovery Research
Curated by ChEMBL
Syntex Discovery Research
Curated by ChEMBL
Affinity DataKi: 1.63E+3nMAssay Description:Binding affinity for cholesterol 17-alpha-hydroxylaseMore data for this Ligand-Target Pair
Affinity DataIC50: 0.260nMAssay Description:Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 0.440nMAssay Description:Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 0.470nMAssay Description:Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 0.590nMAssay Description:Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Antagonist activity at human recombinant CCR5 by cell-cell fusion assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In vitro inhibitory concentration against Methionyl-tRNA synthetase in a pyrophosphate exchange assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Inhibition of CCR5 by cell-cell fusion inhibition assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Antagonist activity at human recombinant CCR5 by cell-cell fusion assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Antagonist activity at human recombinant CCR5 by cell-cell fusion assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Binding affinity at CCR5 receptor by radiolabeled RANTES binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMpH: 2.0Assay Description:The reactions were performed in 96-well Packard 384-well Packard Optiplates. After 10 min of incubation, the reaction was stopped by addition of a co...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Binding affinity at CCR5 receptor by radiolabeled RANTES binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Antagonist activity at human recombinant CCR5 by cell-cell fusion assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Binding affinity at CCR5 receptor by radiolabeled RANTES binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Binding affinity at CCR5 receptor by radiolabeled RANTES binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Binding affinity at CCR5 receptor by radiolabeled RANTES binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Binding affinity at CCR5 receptor by radiolabelled RANTES binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Binding affinity at CCR5 receptor by radiolabeled RANTES binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Binding affinity at CCR5 receptor by radiolabeled RANTES binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 7.80nMAssay Description:Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Binding affinity at CCR5 receptor by radiolabeled RANTES binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMpH: 7.9 T: 2°CAssay Description:The reactions were performed in 96-well Packard 384-well Packard Optiplates. After 10 min of incubation, the reaction was stopped by addition of a co...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Binding affinity at CCR5 receptor by radiolabelled RANTES binding assayMore data for this Ligand-Target Pair

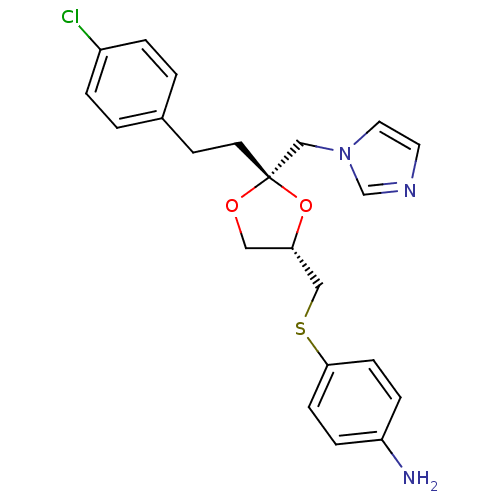
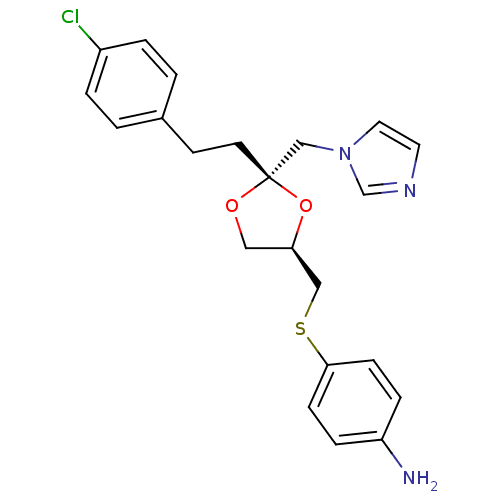
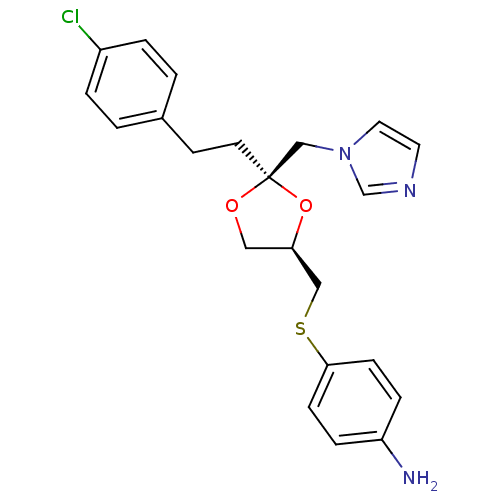
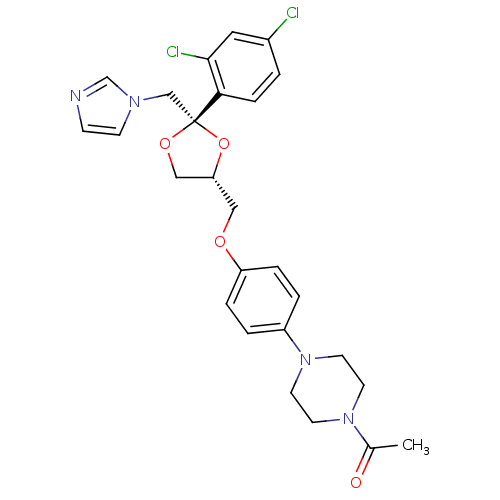




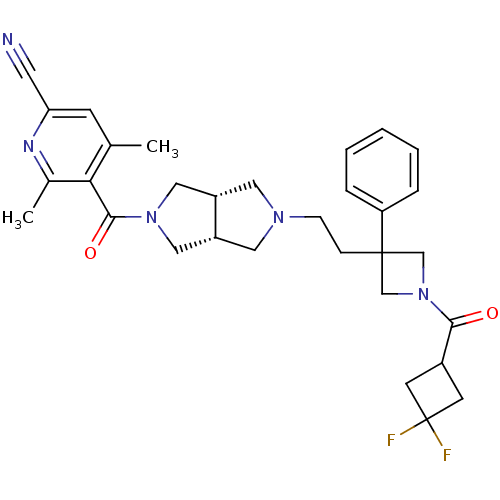

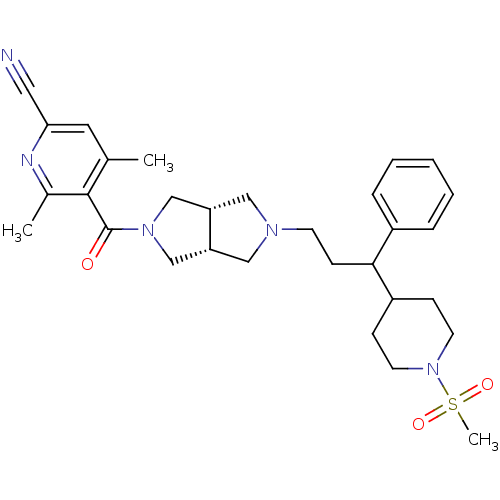

 3D Structure (crystal)
3D Structure (crystal)