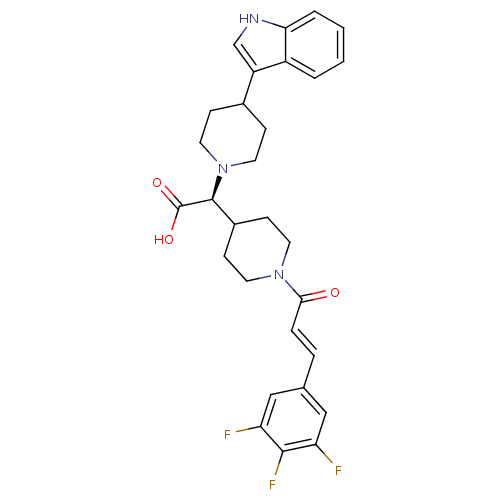
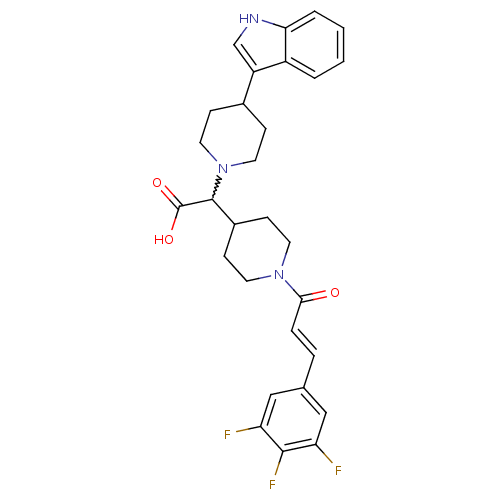
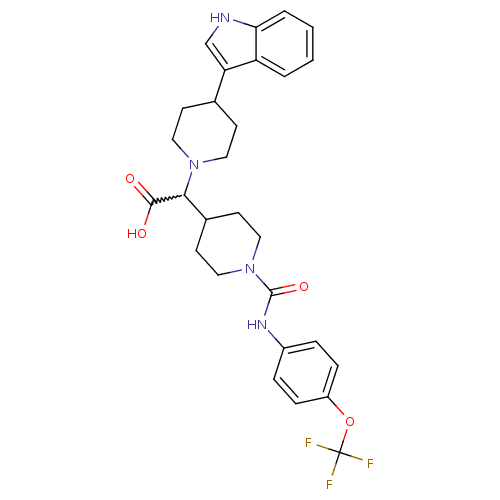
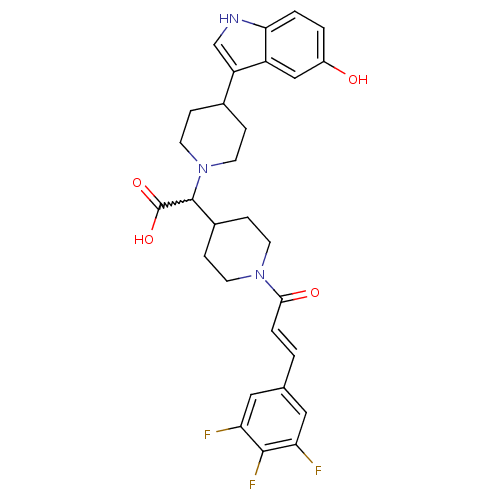
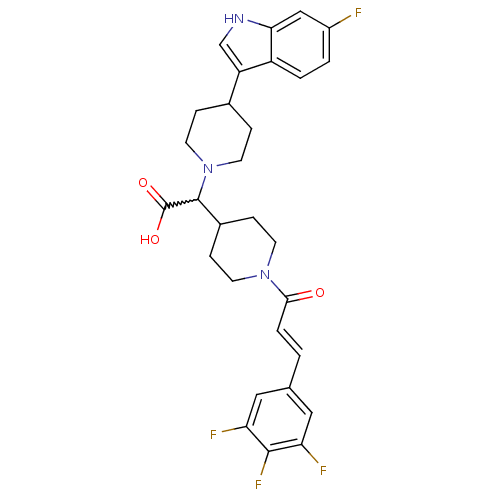
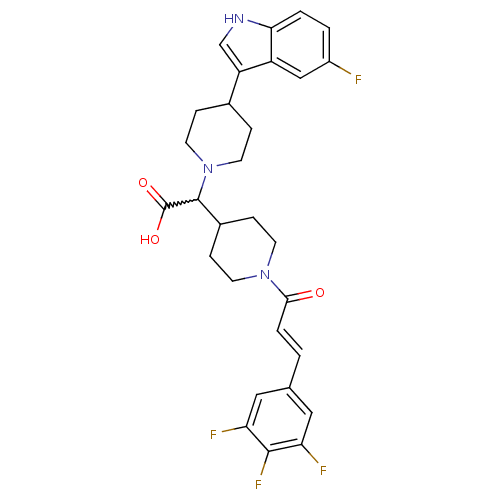
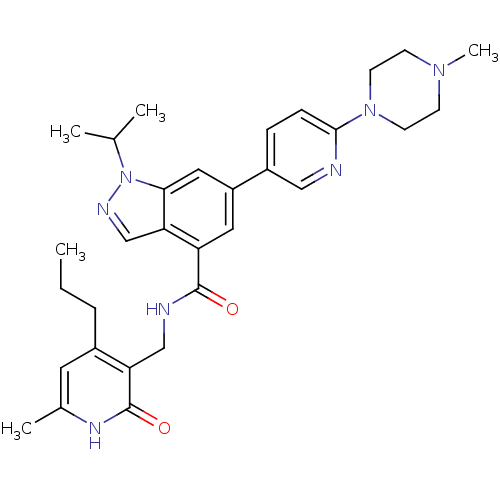
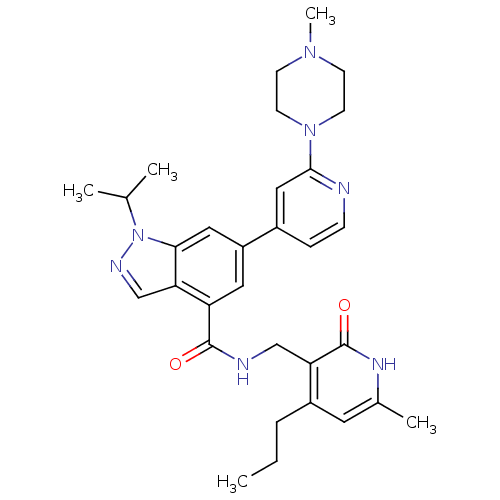
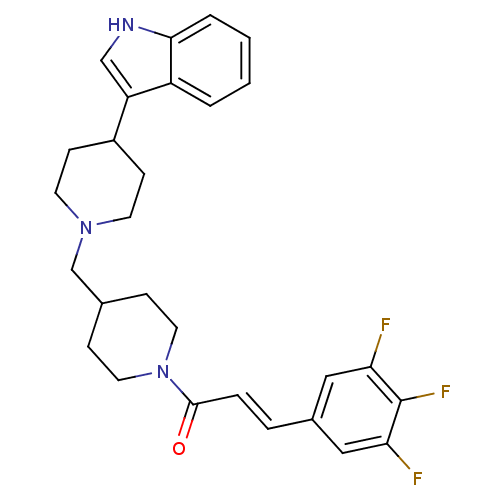
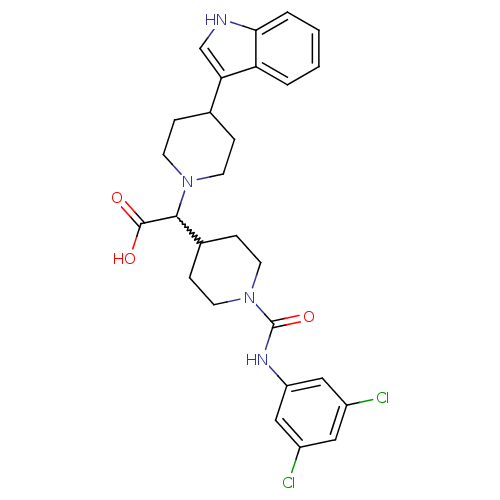
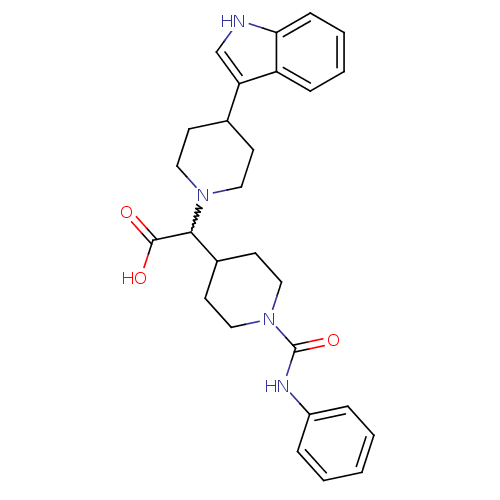
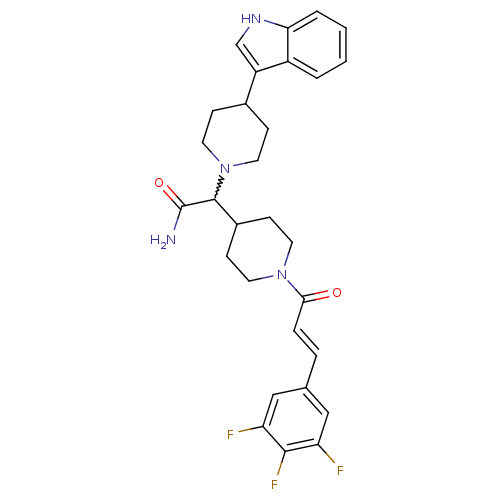
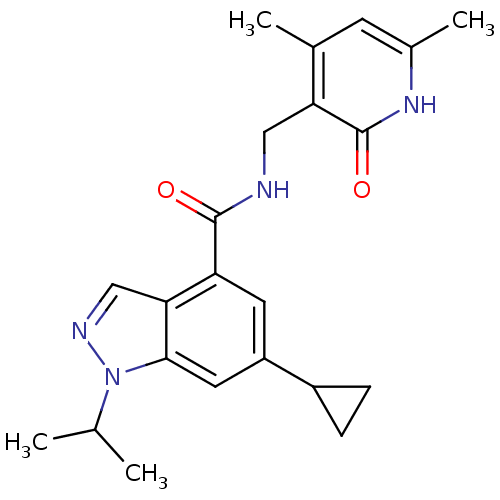
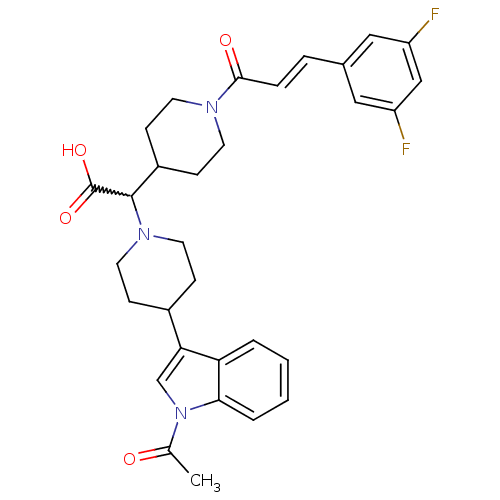
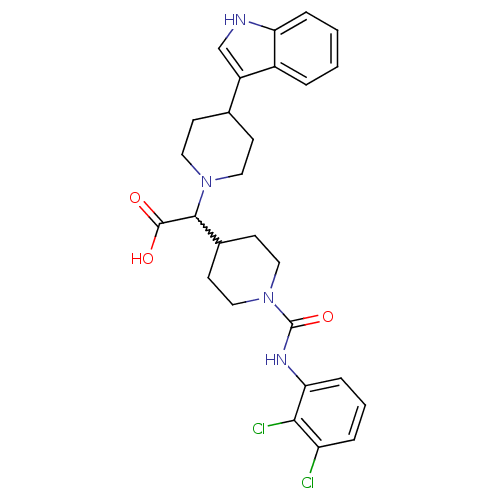
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Antagonist activity at CCR2 expressed in THP1 cells assessed as MCP1-induced calcium fluxMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 2.5nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 6.30nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 7.70nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 16.7nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
Affinity DataIC50: 79nMAssay Description:Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysisMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 115nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
Affinity DataIC50: 174nMAssay Description:Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysisMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 320nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
Affinity DataIC50: 324nMAssay Description:Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysisMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 470nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 660nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 720nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysisMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Mus musculus)
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Displacement of [125I]-mouse MCP1 from CCR2 in mouse WEHI265.1 cellsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Mus musculus)
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Displacement of [125I]-rat MCP1 from rat CCR2 receptor in monocytesMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Mus musculus)
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Displacement of [125I]-mouse MCP1 from CCR2 in mouse peripheral blood monocytesMore data for this Ligand-Target Pair
Affinity DataIC50: 2.51E+3nMAssay Description:Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+3nMAssay Description:Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysisMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 3.30E+3nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 3.40E+3nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
Affinity DataIC50: 4.50E+3nMAssay Description:Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysisMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 6.80E+3nMAssay Description:Inhibition of human CCR2More data for this Ligand-Target Pair
Affinity DataIC50: 1.06E+4nMAssay Description:Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 4.90E+4nMAssay Description:Inhibition of SET7 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.40E+4nMAssay Description:Inhibition of PRMT3 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 6.30E+4nMAssay Description:Inhibition of SET7 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 7.40E+4nMAssay Description:Inhibition of PRMT3 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of SUV39H1 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of SUV39H1 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of SETMAR using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 minsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of SETMAR using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 minsMore data for this Ligand-Target Pair