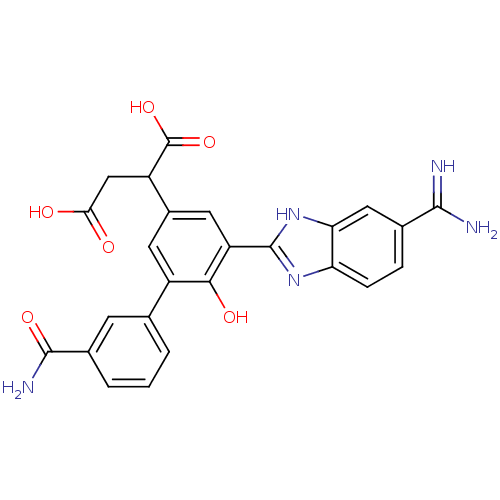
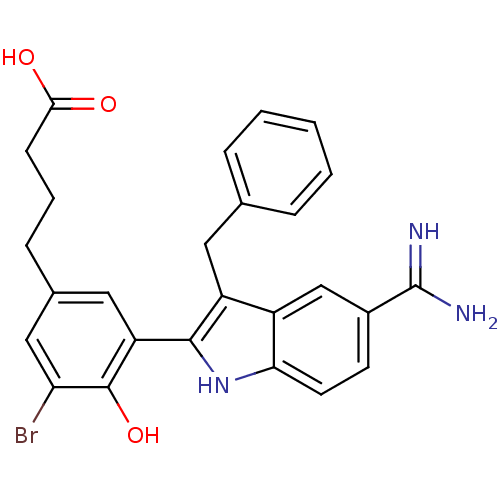
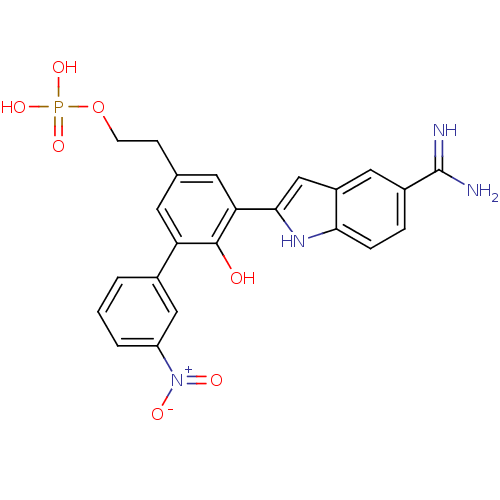
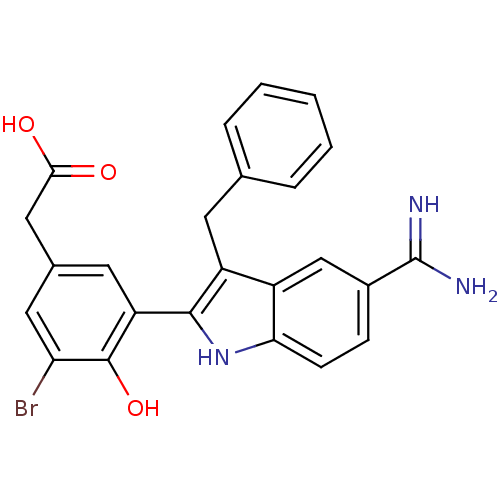
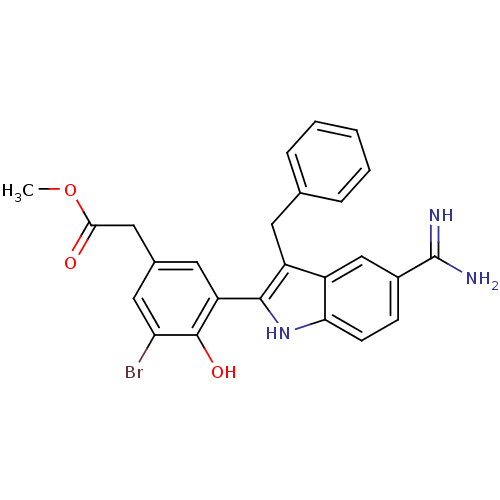
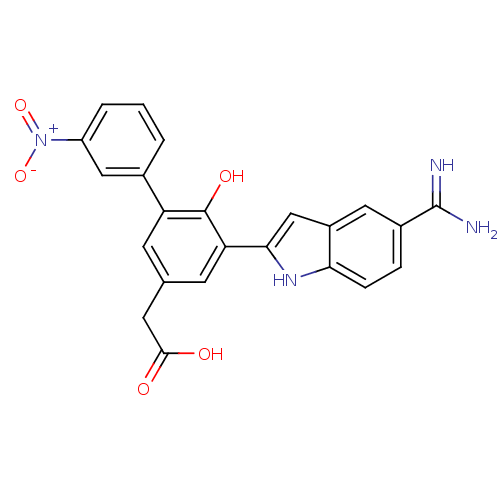
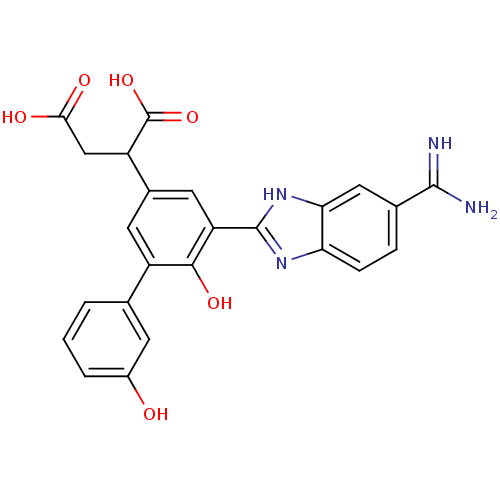
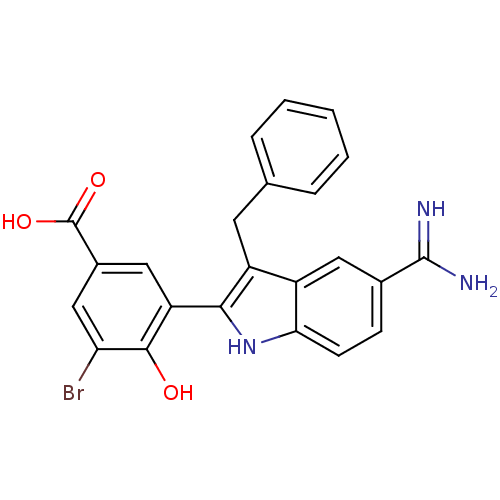
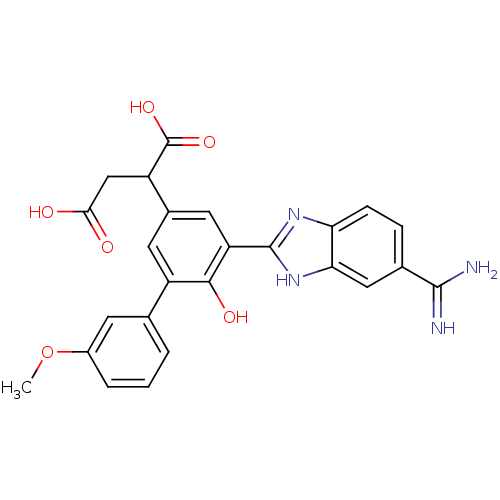
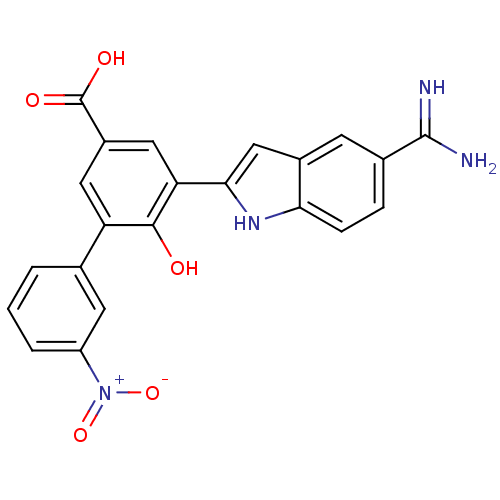
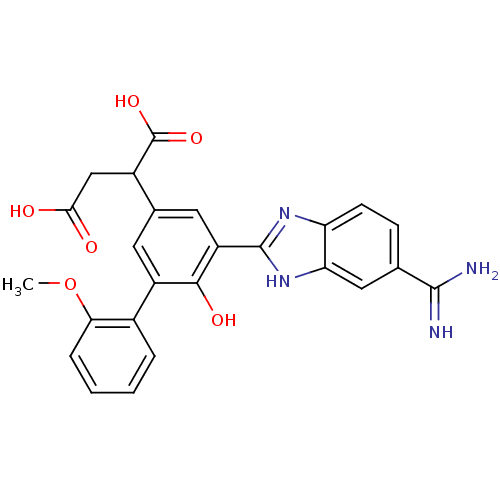
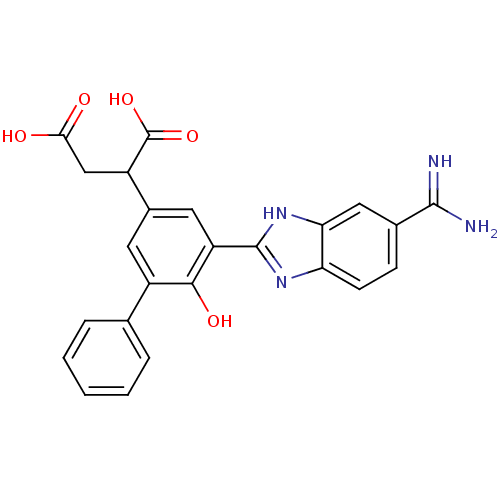
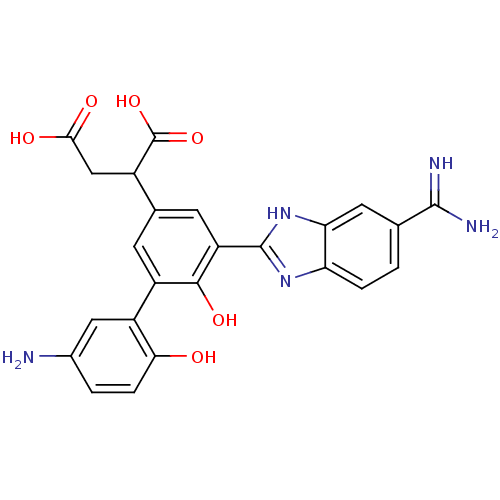
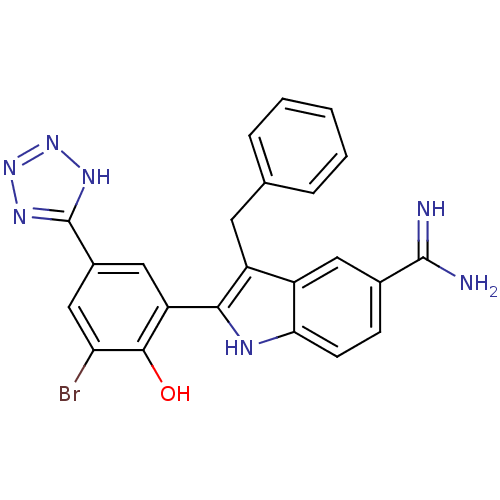
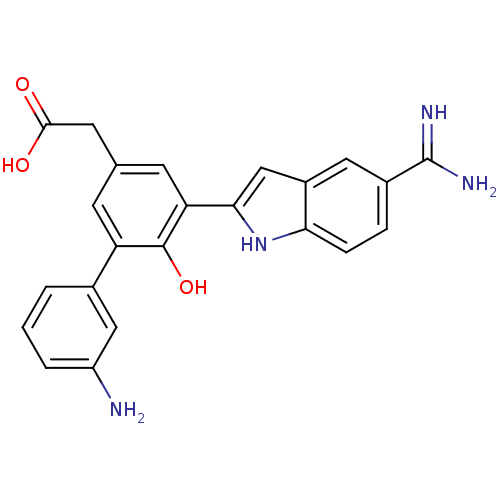
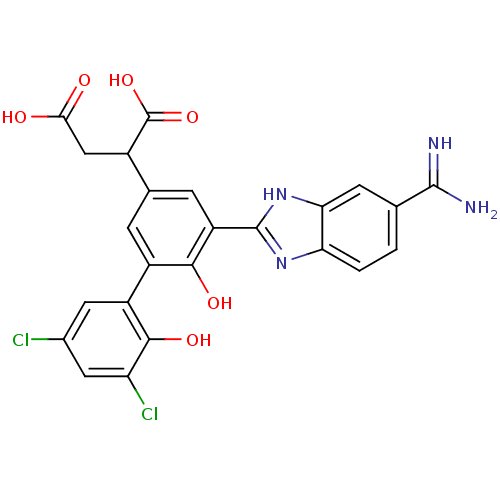
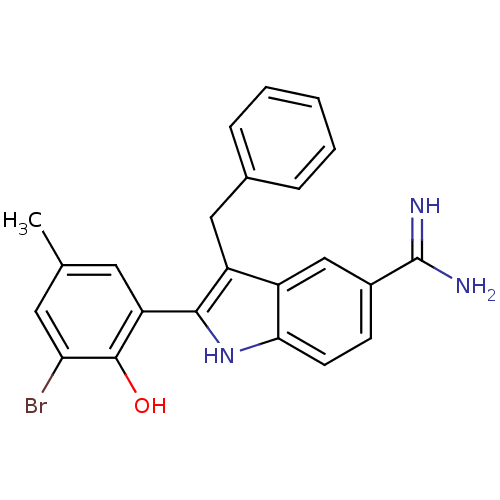
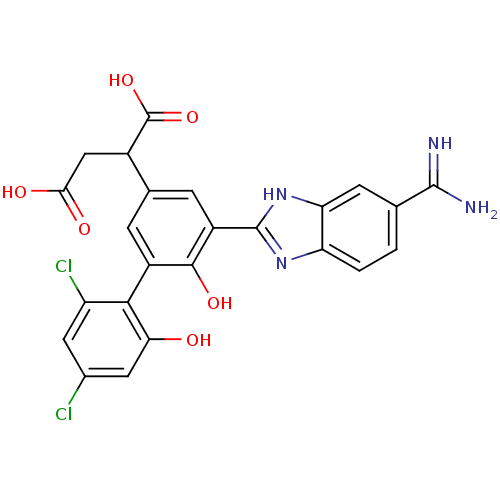
TargetCoagulation factor VII/Tissue factor(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.0780nMAssay Description:Binding affinity for factor VIIa/TFMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 0.460nMAssay Description:Binding affinity of the compound towards urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinity to plasma kallikreinMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinity of the compound towards Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Inhibitory activity against Coagulation factor X in human plasmaMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Inhibitory activity against Coagulation factor X in human plasmaMore data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Inhibitory activity against Coagulation factor X in human plasmaMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Inhibitory activity against Coagulation factor X in human plasmaMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Inhibitory activity against Coagulation factor X in human plasmaMore data for this Ligand-Target Pair
TargetCoagulation factor VII/Tissue factor(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Binding affinity for factor VIIa/TFMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity to plasma kallikreinMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Inhibitory activity against Coagulation factor X in human plasmaMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Inhibitory activity against Coagulation factor X in human plasmaMore data for this Ligand-Target Pair
TargetCoagulation factor VII/Tissue factor(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Binding affinity for factor VIIa/TFMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity to plasma kallikreinMore data for this Ligand-Target Pair
Affinity DataKi: 3.20nMAssay Description:Binding affinity of the compound towards PlasminMore data for this Ligand-Target Pair
Affinity DataKi: 4nM ΔG°: -47.5kJ/molepH: 7.4 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Binding affinity to plasma kallikreinMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Inhibitory activity against Coagulation factor X in human plasmaMore data for this Ligand-Target Pair
Affinity DataKi: 4.20nMAssay Description:Binding affinity of the compound towards Coagulation factor IIMore data for this Ligand-Target Pair
Affinity DataKi: 4.70nMAssay Description:Binding affinity of the compound towards TrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Binding affinity to plasma kallikreinMore data for this Ligand-Target Pair
TargetCoagulation factor VII/Tissue factor(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Binding affinity for factor VIIa/TFMore data for this Ligand-Target Pair
Affinity DataKi: 5.40nM ΔG°: -46.7kJ/molepH: 7.4 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 6nM ΔG°: -46.5kJ/molepH: 7.4 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Binding affinity to plasma kallikreinMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Binding affinity to plasma kallikreinMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Binding affinity to plasma kallikreinMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Binding affinity to plasma kallikreinMore data for this Ligand-Target Pair
Affinity DataKi: 9nM ΔG°: -45.5kJ/molepH: 7.4 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Inhibitory activity against Coagulation factor X in human plasmaMore data for this Ligand-Target Pair
Affinity DataKi: 10nM ΔG°: -45.2kJ/molepH: 7.4 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Inhibitory activity against Coagulation factor X in human plasmaMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Binding affinity to plasma kallikreinMore data for this Ligand-Target Pair
Affinity DataKi: 12nM ΔG°: -44.8kJ/molepH: 7.4 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
TargetCoagulation factor VII/Tissue factor(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Binding affinity for factor VIIa/TFMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Binding affinity to plasma kallikreinMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Binding affinity to plasma kallikreinMore data for this Ligand-Target Pair
Affinity DataKi: 13nM ΔG°: -44.6kJ/molepH: 7.4 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 14nM ΔG°: -44.4kJ/molepH: 7.4 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Binding affinity to plasma kallikreinMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibitory activity against Coagulation factor X in human plasmaMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibitory activity against Coagulation factor X in human plasmaMore data for this Ligand-Target Pair
Affinity DataKi: 21nM ΔG°: -43.4kJ/molepH: 7.4 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 22nM ΔG°: -43.3kJ/molepH: 7.4 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 22nM ΔG°: -43.3kJ/molepH: 7.4 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 25nM ΔG°: -43.0kJ/molepH: 7.4 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Inhibitory activity against thrombin(fIIa) in human plasmaMore data for this Ligand-Target Pair
Affinity DataKi: 27nM ΔG°: -42.8kJ/molepH: 7.4 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 27nMAssay Description:Binding affinity to plasma kallikreinMore data for this Ligand-Target Pair