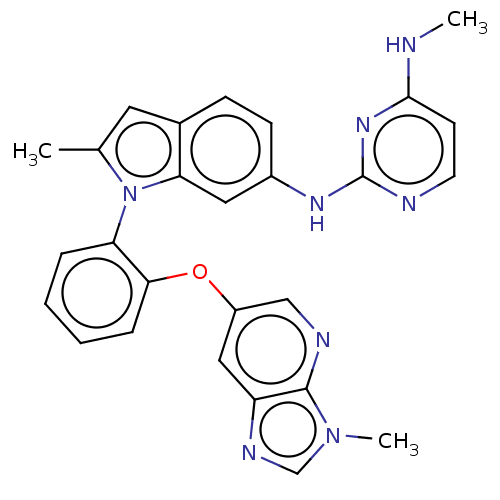
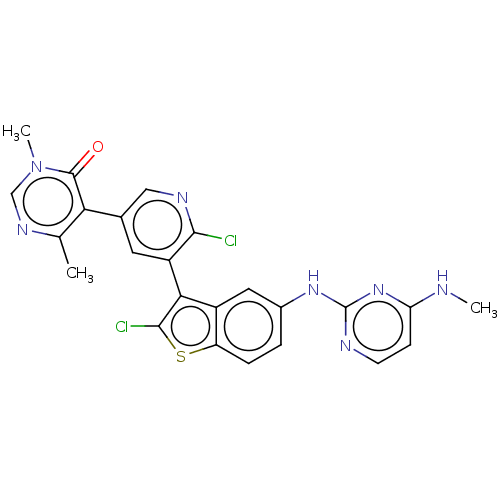
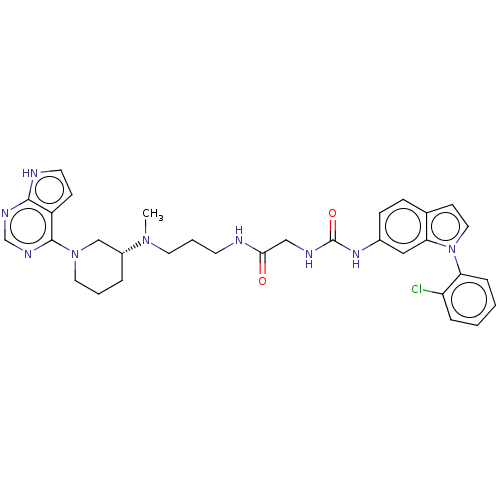
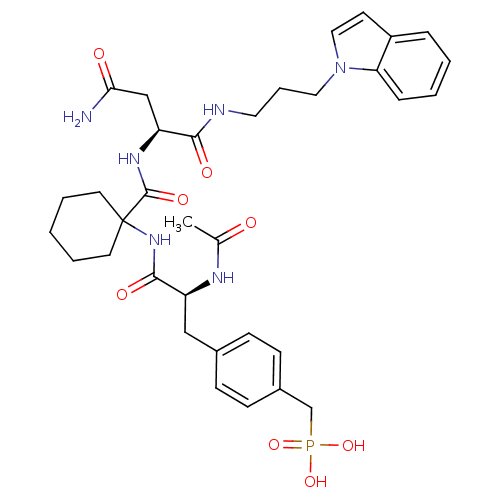
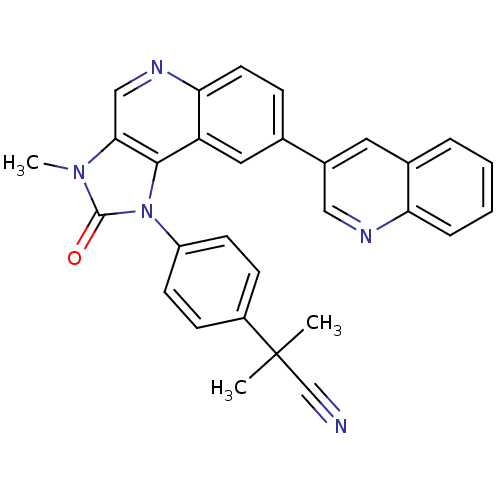
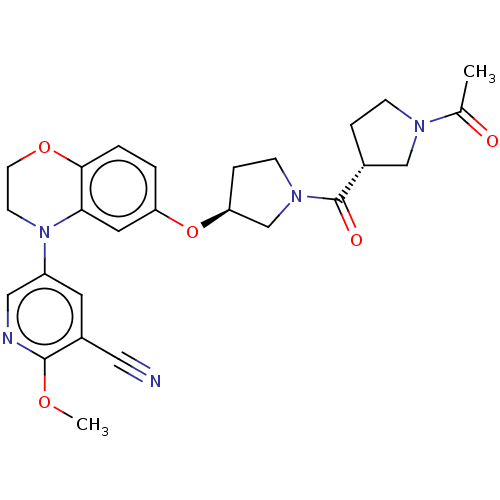
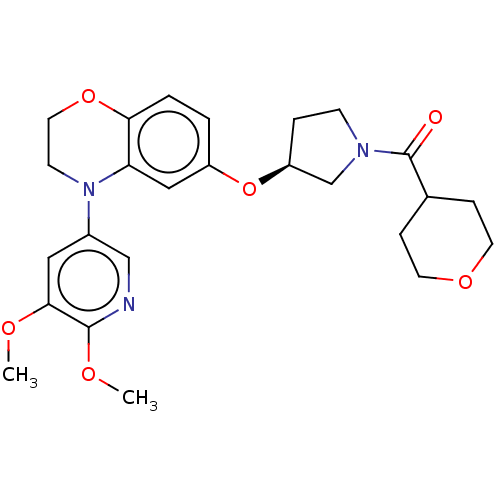
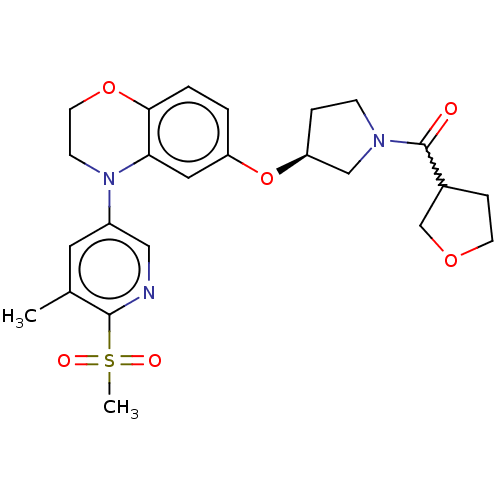
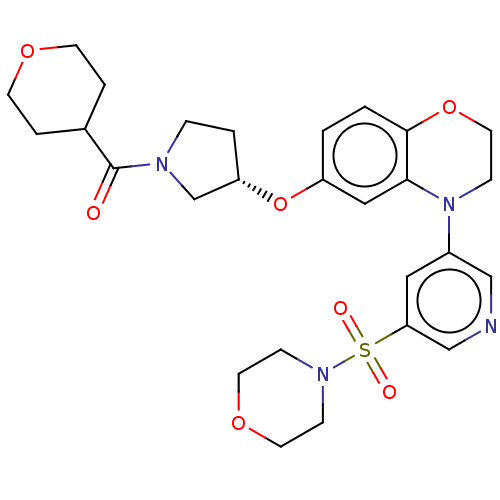
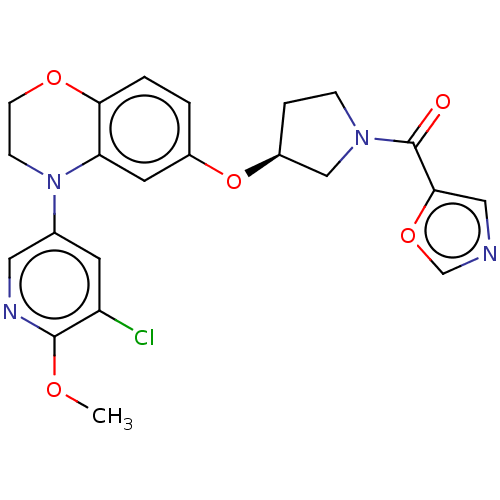
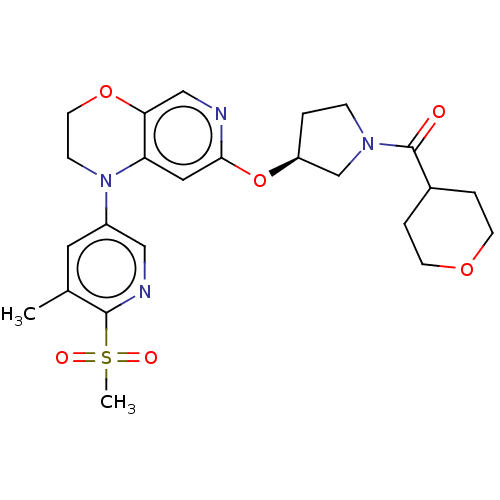
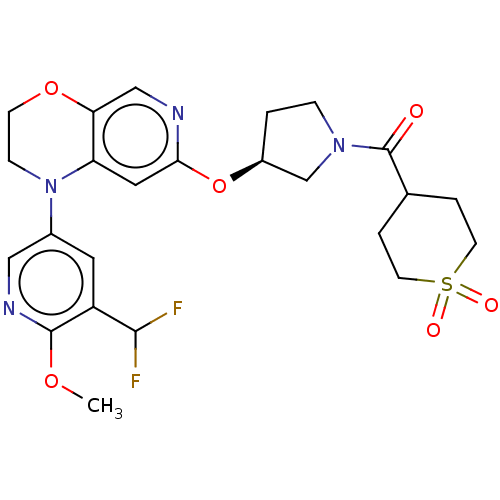
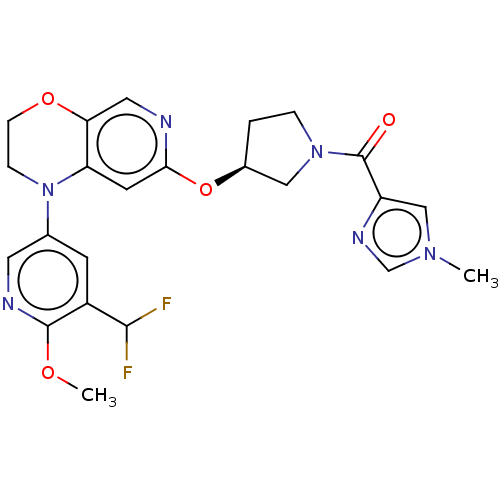
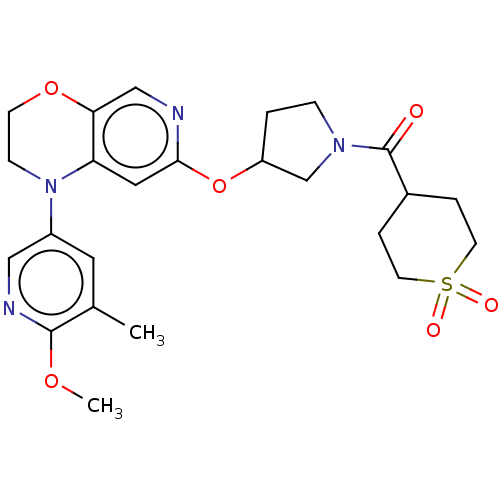
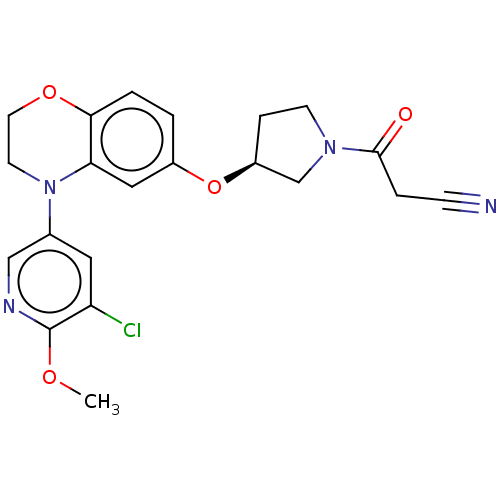
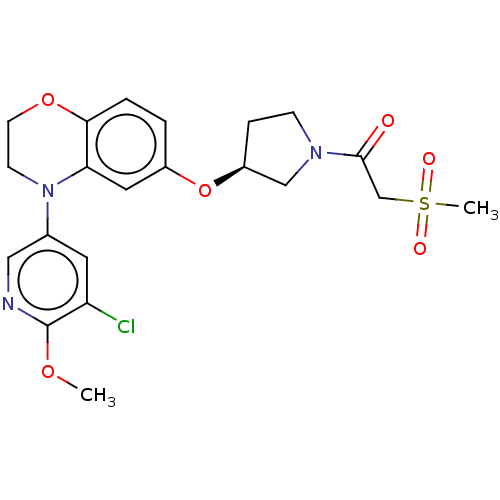
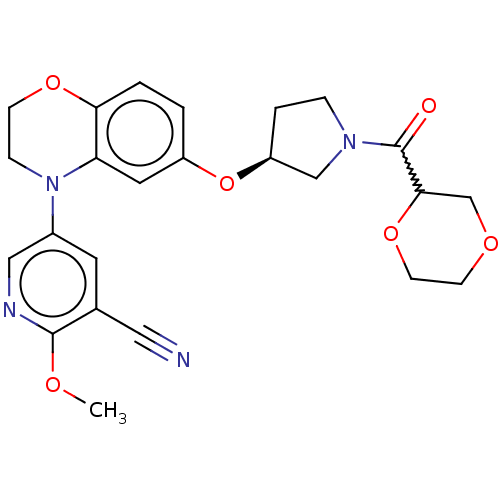
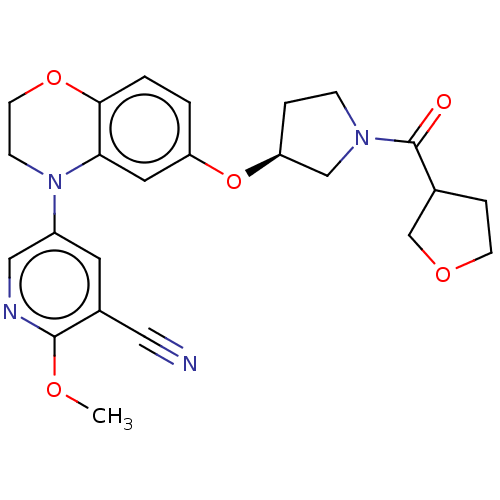
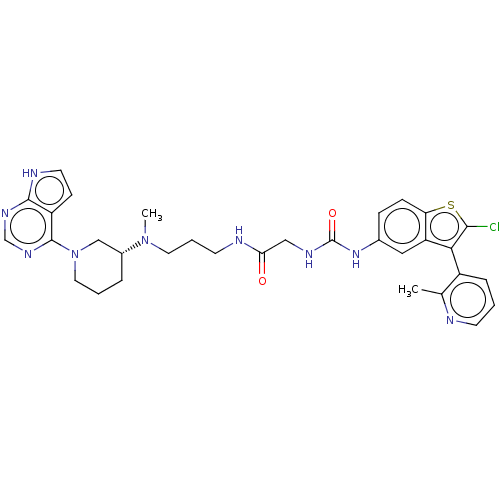
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 0.00200nMAssay Description:Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by...More data for this Ligand-Target Pair
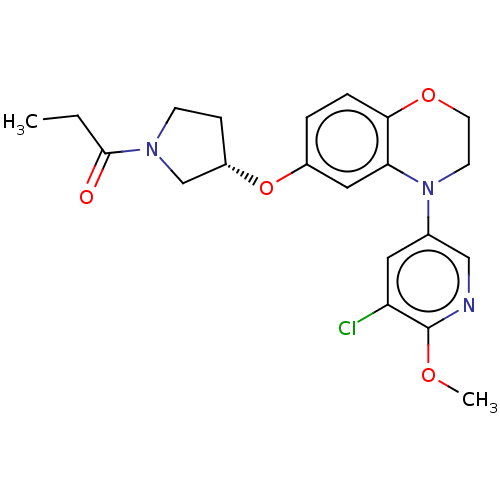
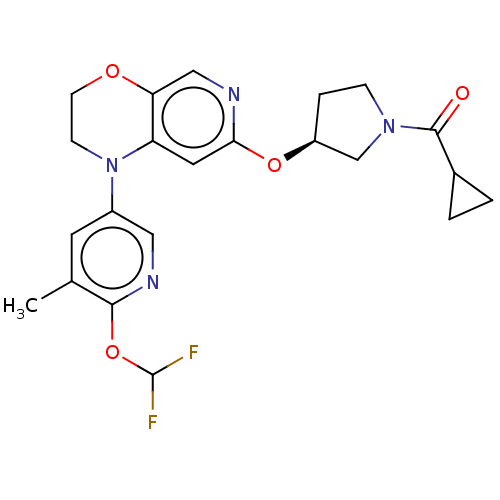
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 0.0120nMAssay Description:Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by...More data for this Ligand-Target Pair
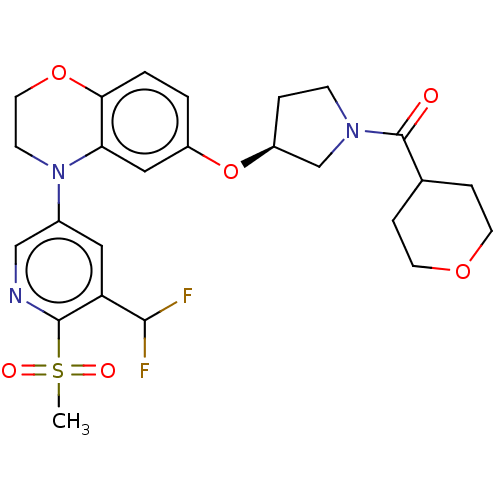
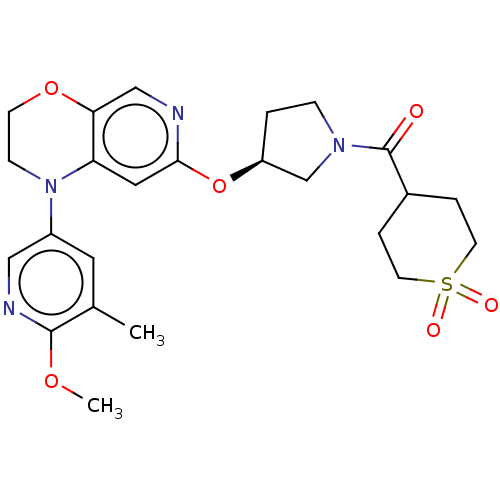
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 0.0800nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
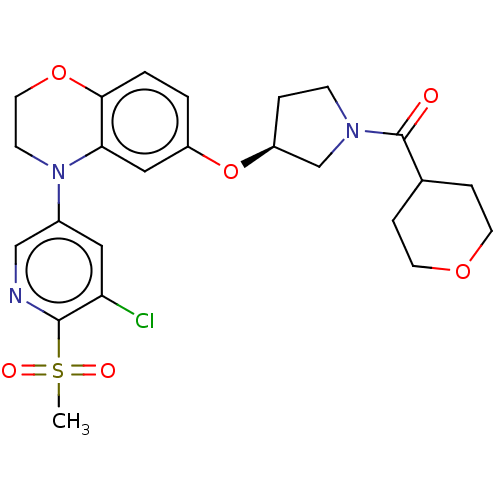
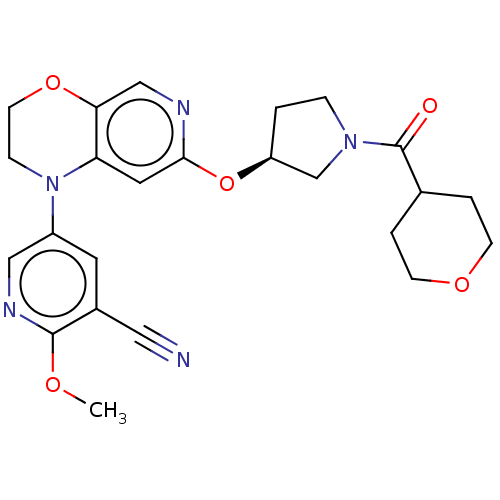
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataKi: 0.360nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00400nMAssay Description:Inhibition of binding to Growth factor receptor bound protein 2 (Grb2) SH2 domainMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: <0.100nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: <0.100nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.150nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of Grb-SH2 domain binding to phosphorylated carboxy-terminal intracellular domain of epidermal growth factor receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of Grb-SH2 domain binding to phosphorylated carboxy-terminal intracellular domain of epidermal growth factor receptorMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Inhibition of Grb-SH2 domain binding to phosphorylated carboxy-terminal intracellular domain of epidermal growth factor receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of binding to Grb2 SH2 domainMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Inhibition of Grb-SH2 domain binding to phosphorylated carboxy-terminal intracellular domain of epidermal growth factor receptorMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a...More data for this Ligand-Target Pair
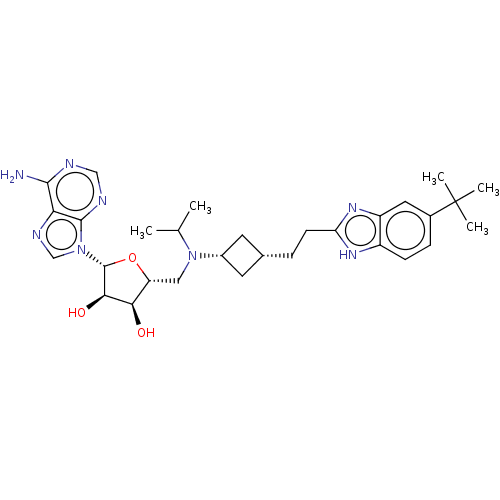
TargetSerine/threonine-protein kinase mTOR(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.90nMAssay Description:Displacement of Alexa Fluor labelled Tracer-314 from human N-terminal GST-tagged mTOR incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: <3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: <3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: <3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: <3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: <3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: <3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: <3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: <3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: <3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: <3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: <3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: <3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: <3nMT: 2°CAssay Description:Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase, H3 lysine-79 specific(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of DOT1L in human HeLa cells assessed as reduction in H3K79me2 level after 72 hrs by ELISAMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent

 3D Structure (crystal)
3D Structure (crystal)