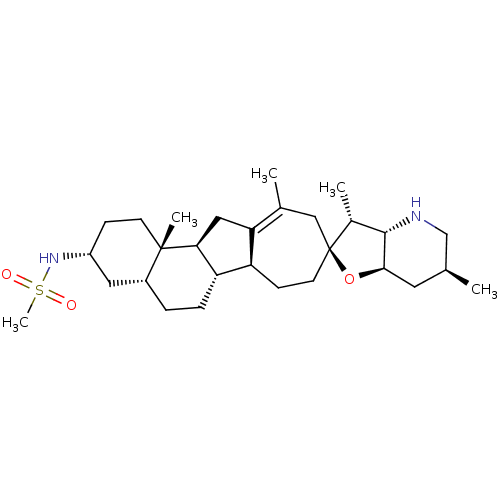
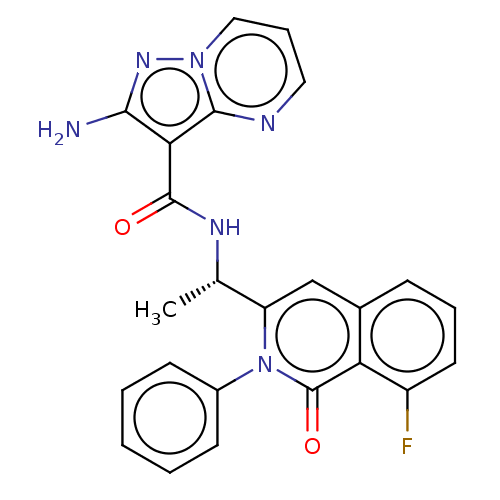
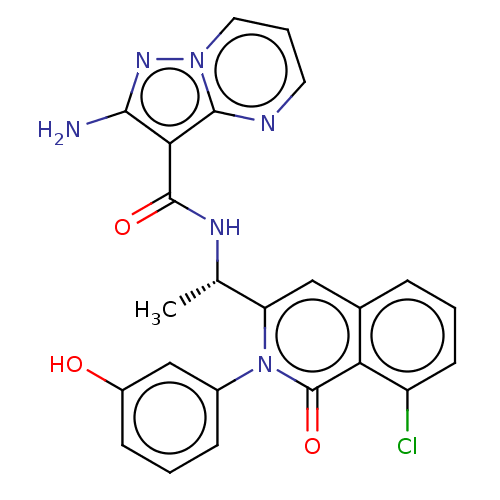
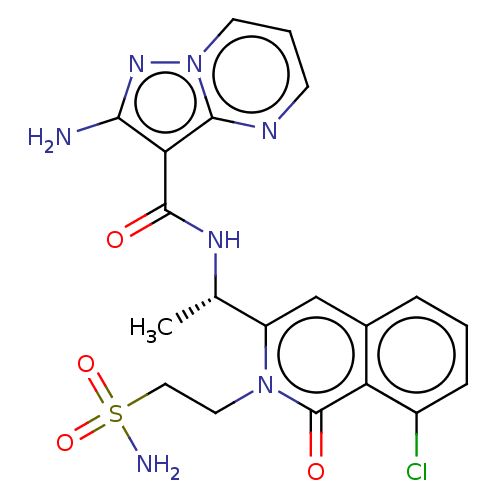
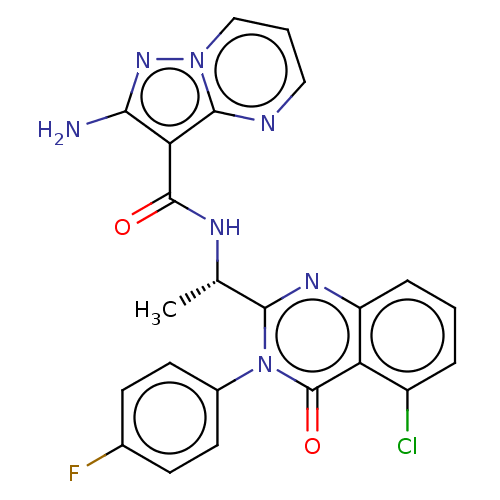
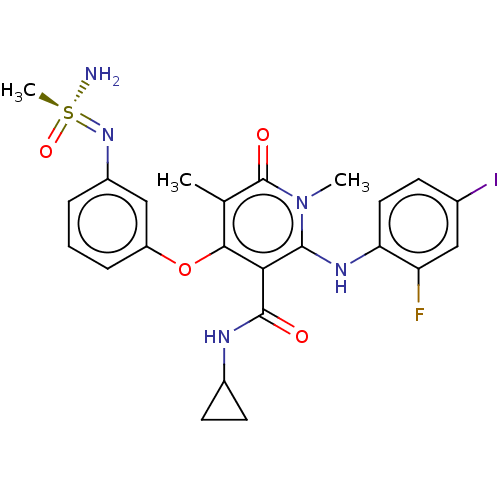
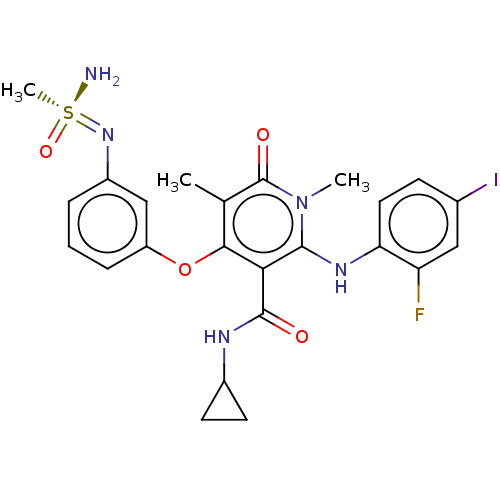
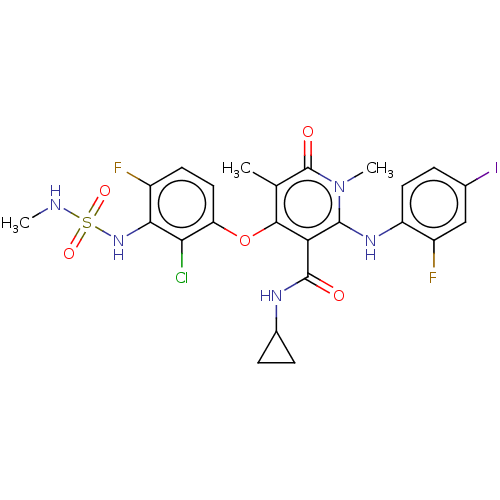
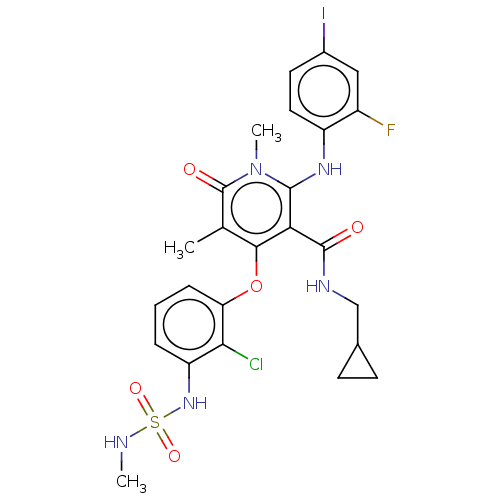
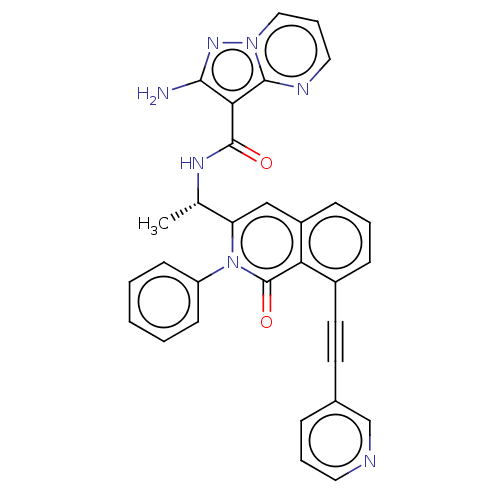
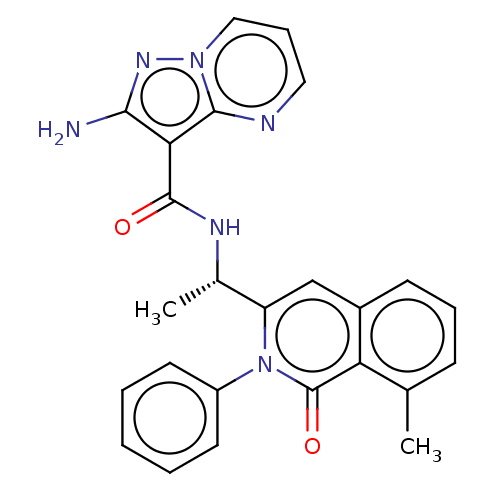
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Mus musculus (Mouse))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of PI3Kgamma in C5a-stimulated mouse RAW264.7 cells assessed as reduction in AKT phosphorylation at S473 incubated for 30 mins followed by...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of human recombinant SMO expressed in mouse C3H10T1/2 cells assessed as inhibition of association of BODIPY-cyclopamineMore data for this Ligand-Target Pair
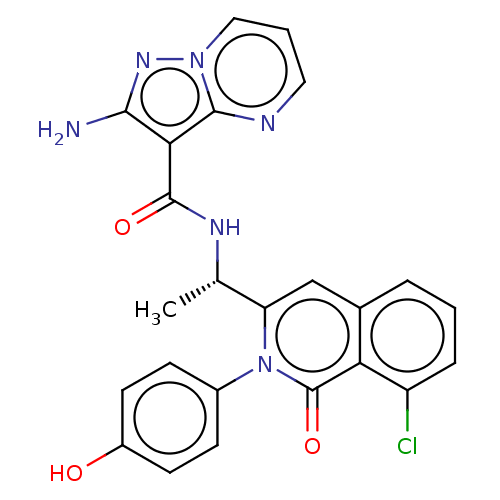
TargetPhosphatidylinositol 3-kinase regulatory subunit beta(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 5.5nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
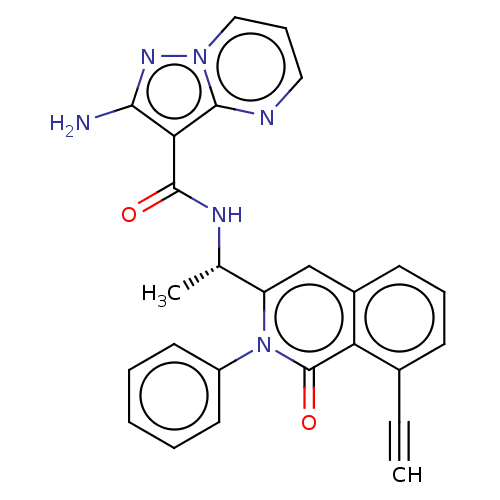
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1...More data for this Ligand-Target Pair
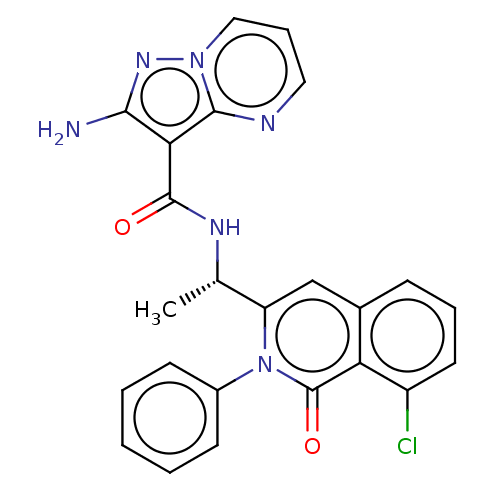
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit beta(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 55nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit beta(Homo sapiens (Human))
Infinity Pharmaceuticals
US Patent
Infinity Pharmaceuticals
US Patent
Affinity DataIC50: 55nMAssay Description:Class I PI3-Ks can be either purchased (p110α/p85α, p110β/p85α, p110δ/p85α from Upstate, and p110γ from Sigma) or ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 75nMAssay Description:Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1...More data for this Ligand-Target Pair
TargetInhibitor of nuclear factor kappa-B kinase subunit alpha(Homo sapiens (Human))
Millennium Pharmaceuticals
Curated by ChEMBL
Millennium Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibitory activity against IkappaB kinase(IKK) isolated from HeLa cells activated with recombinant MEEK1 in an ELISA phosphorylaton assay.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1/Serine/threonine-protein kinase B-raf(Homo sapiens (Human))
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataIC50: <100nMAssay Description:Table 3: Compound effects on binding affinity of MEK to BRAF or CRAF were followed by surface plasmon resonance (SPR) with single-cycle kinetic analy...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1/Serine/threonine-protein kinase B-raf(Homo sapiens (Human))
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataIC50: <100nMAssay Description:Table 3: Compound effects on binding affinity of MEK to BRAF or CRAF were followed by surface plasmon resonance (SPR) with single-cycle kinetic analy...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1...More data for this Ligand-Target Pair
TargetInhibitor of nuclear factor kappa-B kinase subunit alpha(Homo sapiens (Human))
Millennium Pharmaceuticals
Curated by ChEMBL
Millennium Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibitory activity against IkappaB kinase(IKK) isolated from HeLa cells activated with recombinant MEEK1 in an ELISA phosphorylaton assay.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1/Serine/threonine-protein kinase B-raf(Homo sapiens (Human))
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataIC50: <100nMAssay Description:Table 3: Compound effects on binding affinity of MEK to BRAF or CRAF were followed by surface plasmon resonance (SPR) with single-cycle kinetic analy...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1/Serine/threonine-protein kinase B-raf(Homo sapiens (Human))
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataIC50: <100nMAssay Description:Table 3: Compound effects on binding affinity of MEK to BRAF or CRAF were followed by surface plasmon resonance (SPR) with single-cycle kinetic analy...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1/Serine/threonine-protein kinase B-raf(Homo sapiens (Human))
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataIC50: <100nMAssay Description:Table 3: Compound effects on binding affinity of MEK to BRAF or CRAF were followed by surface plasmon resonance (SPR) with single-cycle kinetic analy...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1/Serine/threonine-protein kinase B-raf(Homo sapiens (Human))
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataIC50: <100nMAssay Description:Table 3: Compound effects on binding affinity of MEK to BRAF or CRAF were followed by surface plasmon resonance (SPR) with single-cycle kinetic analy...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1/Serine/threonine-protein kinase B-raf(Homo sapiens (Human))
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataIC50: <100nMAssay Description:Table 3: Compound effects on binding affinity of MEK to BRAF or CRAF were followed by surface plasmon resonance (SPR) with single-cycle kinetic analy...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1/Serine/threonine-protein kinase B-raf(Homo sapiens (Human))
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataIC50: <100nMAssay Description:Table 3: Compound effects on binding affinity of MEK to BRAF or CRAF were followed by surface plasmon resonance (SPR) with single-cycle kinetic analy...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1/Serine/threonine-protein kinase B-raf(Homo sapiens (Human))
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataIC50: <100nMAssay Description:Table 3: Compound effects on binding affinity of MEK to BRAF or CRAF were followed by surface plasmon resonance (SPR) with single-cycle kinetic analy...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1/Serine/threonine-protein kinase B-raf(Homo sapiens (Human))
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataIC50: <100nMAssay Description:Table 3: Compound effects on binding affinity of MEK to BRAF or CRAF were followed by surface plasmon resonance (SPR) with single-cycle kinetic analy...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1/Serine/threonine-protein kinase B-raf(Homo sapiens (Human))
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataIC50: <100nMAssay Description:Table 3: Compound effects on binding affinity of MEK to BRAF or CRAF were followed by surface plasmon resonance (SPR) with single-cycle kinetic analy...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1/Serine/threonine-protein kinase B-raf(Homo sapiens (Human))
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataIC50: <100nMAssay Description:Table 3: Compound effects on binding affinity of MEK to BRAF or CRAF were followed by surface plasmon resonance (SPR) with single-cycle kinetic analy...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1/Serine/threonine-protein kinase B-raf(Homo sapiens (Human))
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataIC50: <100nMAssay Description:Table 3: Compound effects on binding affinity of MEK to BRAF or CRAF were followed by surface plasmon resonance (SPR) with single-cycle kinetic analy...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1/Serine/threonine-protein kinase B-raf(Homo sapiens (Human))
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataIC50: <100nMAssay Description:Table 3: Compound effects on binding affinity of MEK to BRAF or CRAF were followed by surface plasmon resonance (SPR) with single-cycle kinetic analy...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1/Serine/threonine-protein kinase B-raf(Homo sapiens (Human))
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataIC50: <100nMAssay Description:Table 3: Compound effects on binding affinity of MEK to BRAF or CRAF were followed by surface plasmon resonance (SPR) with single-cycle kinetic analy...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1/Serine/threonine-protein kinase B-raf(Homo sapiens (Human))
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataIC50: <100nMAssay Description:Table 3: Compound effects on binding affinity of MEK to BRAF or CRAF were followed by surface plasmon resonance (SPR) with single-cycle kinetic analy...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1/Serine/threonine-protein kinase B-raf(Homo sapiens (Human))
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataIC50: <100nMAssay Description:Table 3: Compound effects on binding affinity of MEK to BRAF or CRAF were followed by surface plasmon resonance (SPR) with single-cycle kinetic analy...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1/Serine/threonine-protein kinase B-raf(Homo sapiens (Human))
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataIC50: <100nMAssay Description:Table 3: Compound effects on binding affinity of MEK to BRAF or CRAF were followed by surface plasmon resonance (SPR) with single-cycle kinetic analy...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1/Serine/threonine-protein kinase B-raf(Homo sapiens (Human))
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataIC50: <100nMAssay Description:Table 3: Compound effects on binding affinity of MEK to BRAF or CRAF were followed by surface plasmon resonance (SPR) with single-cycle kinetic analy...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1/Serine/threonine-protein kinase B-raf(Homo sapiens (Human))
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataIC50: <100nMAssay Description:Table 3: Compound effects on binding affinity of MEK to BRAF or CRAF were followed by surface plasmon resonance (SPR) with single-cycle kinetic analy...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 1/Serine/threonine-protein kinase B-raf(Homo sapiens (Human))
Ikena Oncology
US Patent
Ikena Oncology
US Patent
Affinity DataIC50: <100nMAssay Description:Table 3: Compound effects on binding affinity of MEK to BRAF or CRAF were followed by surface plasmon resonance (SPR) with single-cycle kinetic analy...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Infinity Pharmaceuticals
Curated by ChEMBL
Infinity Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1...More data for this Ligand-Target Pair

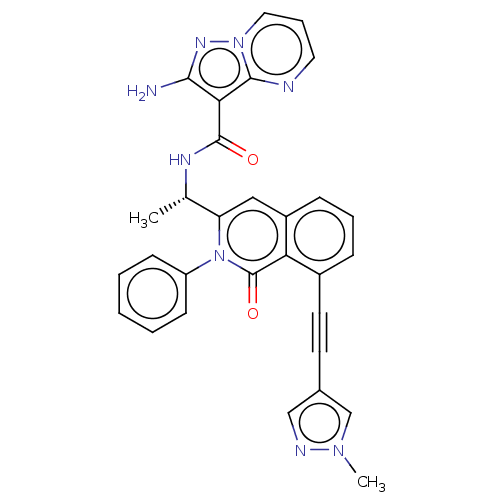
 3D Structure (crystal)
3D Structure (crystal)