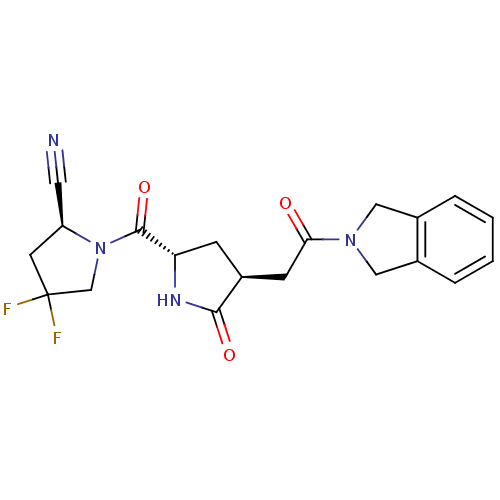
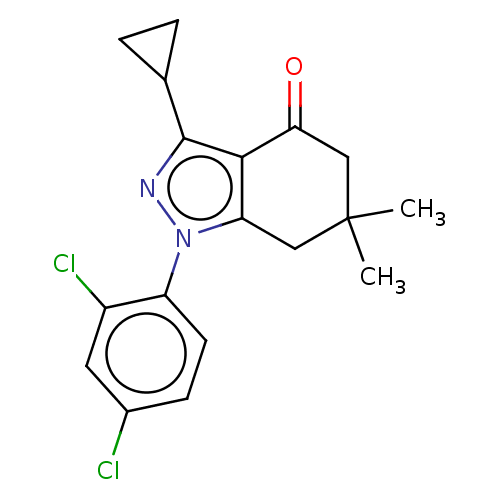
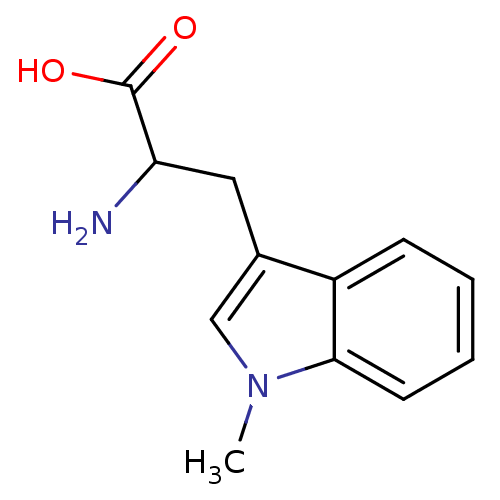
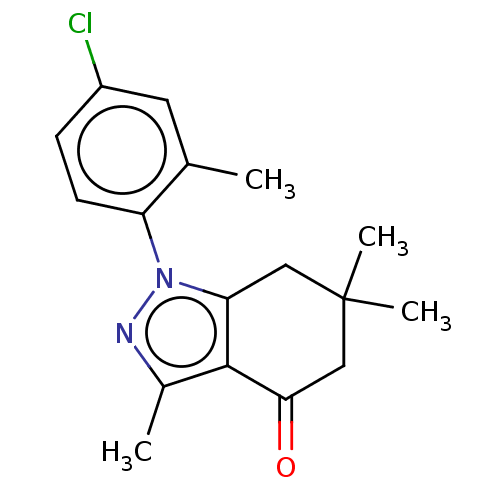
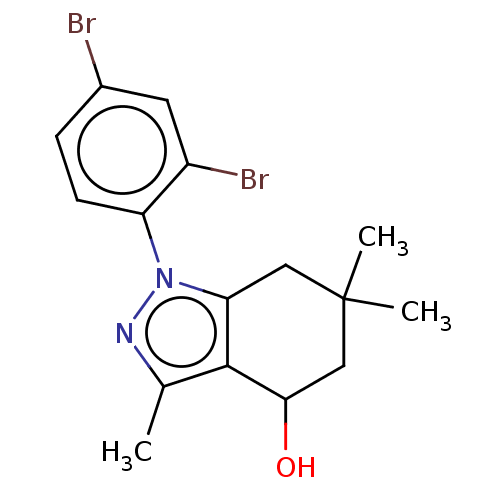
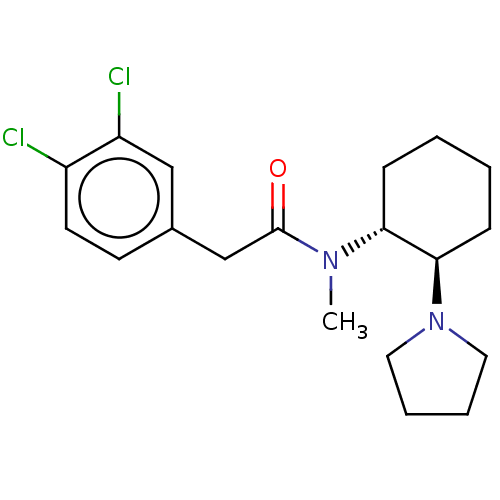
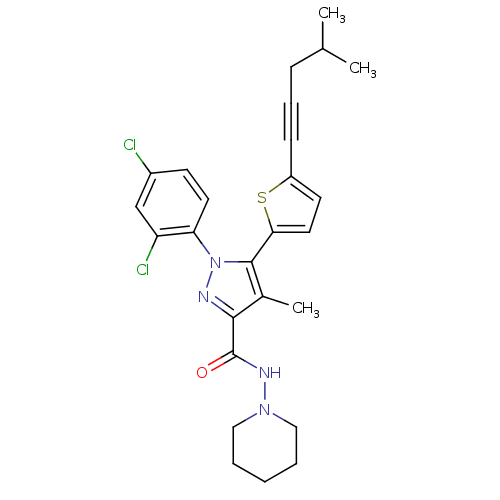
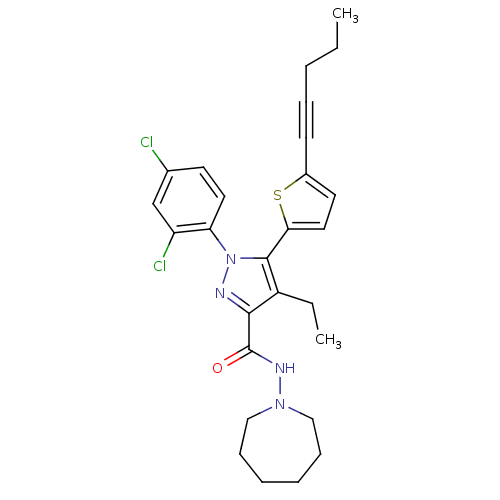
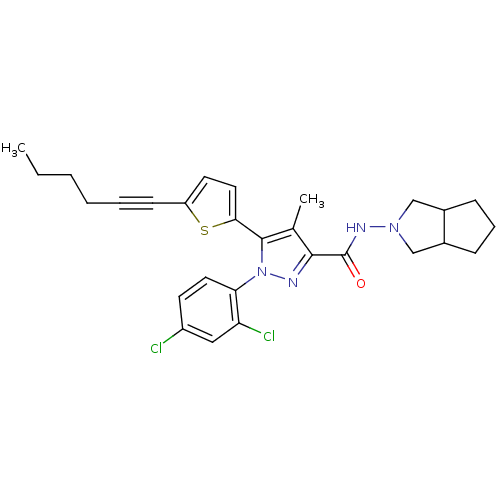
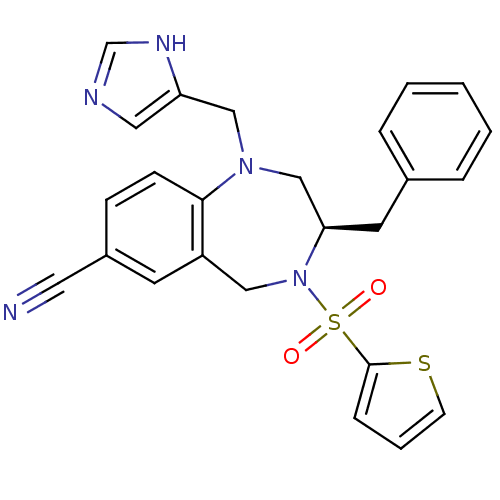
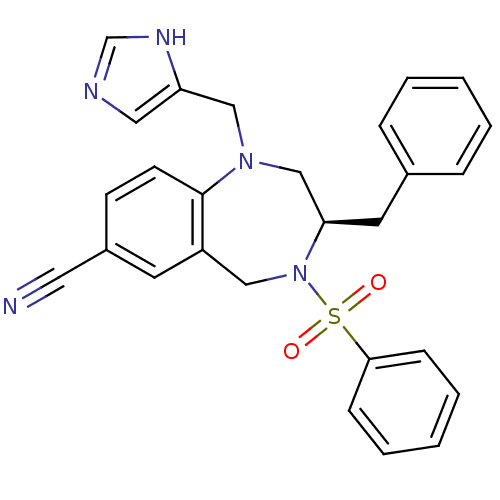
Affinity DataKi: 0.600nMAssay Description:Inhibition of Aurora A kinaseMore data for this Ligand-Target Pair
TargetProlyl endopeptidase FAP(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Competitive inhibition of human recombinant FAP expressed in Hi5 insect cells by Lineweaver-Burke plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Inhibition of Aurora B kinaseMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 minsMore data for this Ligand-Target Pair
Affinity DataKi: 4.60nMAssay Description:Inhibition of Aurora C kinaseMore data for this Ligand-Target Pair
TargetProlyl endopeptidase FAP(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataKi: 7.90nMAssay Description:Competitive inhibition of human recombinant FAP expressed in Hi5 insect cells by Lineweaver-Burke plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibition of Aurora A kinaseMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 26nMAssay Description:Inhibitory activity against VEGFR2More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 26nMAssay Description:Inhibitory activity against VEGFR2More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Mus musculus)
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 28nMAssay Description:Inhibitory activity against Flk1More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 1(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Inhibitory activity against VEGFR1More data for this Ligand-Target Pair
Affinity DataKi: 61nMAssay Description:Inhibition of Aurora C kinaseMore data for this Ligand-Target Pair
Affinity DataKi: 79nMAssay Description:Inhibition of Aurora B kinaseMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataKi: 470nMAssay Description:Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 minsMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataKi: 610nMAssay Description:Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 minsMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataKi: 740nMAssay Description:Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 minsMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataKi: 1.30E+3nMAssay Description:Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 minsMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataKi: 3.93E+3nMAssay Description:Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 minsMore data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataKi: 3.40E+4nMAssay Description:Inhibition of human recombinant IDO using L-tryptophan as substrateMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 minsMore data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 minsMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Agonist activity at human kappa opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP production after 30 mins b...More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Agonist activity at human delta opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP production after 30 mins b...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of FLT3 autophosphorylation in human MV4-11 cells after 2 hrs by Western blot analysisMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assayMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Displacement of [3H]SR141716A from human CB1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetDimer of Protein farnesyltransferase subunit beta(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.35nMAssay Description:Inhibition of purified recombinant human farnesyltransferase (FT)More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetDimer of Protein farnesyltransferase subunit beta(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.53nMAssay Description:Inhibition of purified recombinant human farnesyltransferase (FT)More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMpH: 8.0 T: 2°CAssay Description:The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro...More data for this Ligand-Target Pair
TargetDimer of Protein farnesyltransferase subunit beta(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.77nMAssay Description:Inhibition of purified recombinant human farnesyltransferase (FT)More data for this Ligand-Target Pair
TargetDimer of Protein farnesyltransferase subunit beta(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.77nMAssay Description:Inhibition of purified recombinant human farnesyltransferase (FT)More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assayMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of wild type GST tagged FLT3 kinase (567 to 993) (unknown origin) transfected in insect sf9 cells after 4 hrs by wallac counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of DPP4 (unknown origin)More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 2.60nMAssay Description:Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:Inhibition of human DPP4 expressed in baculovirus systemMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)