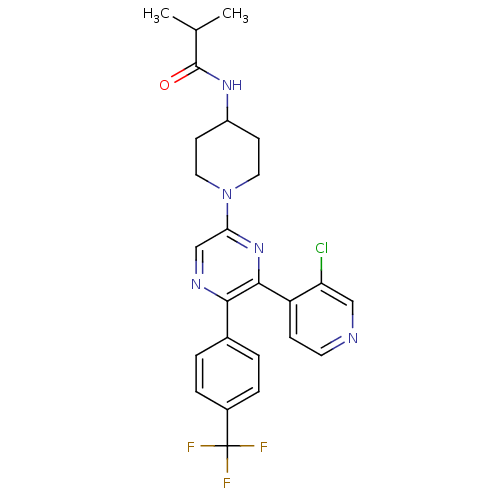
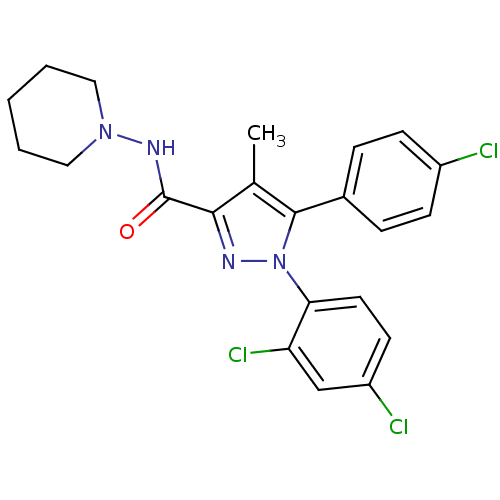
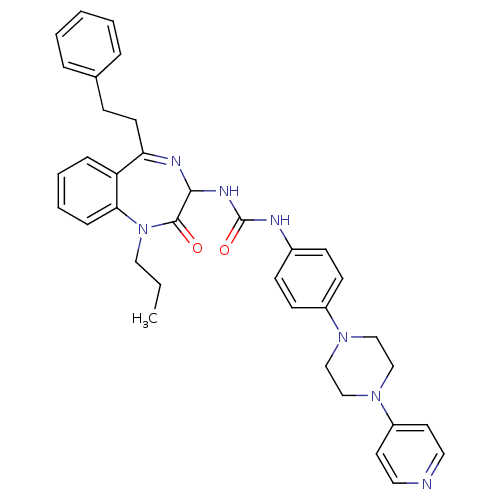
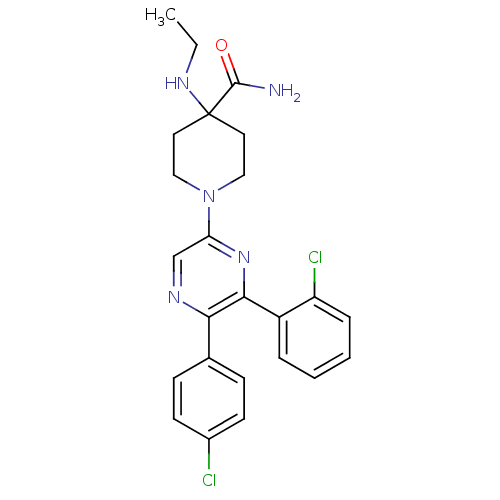
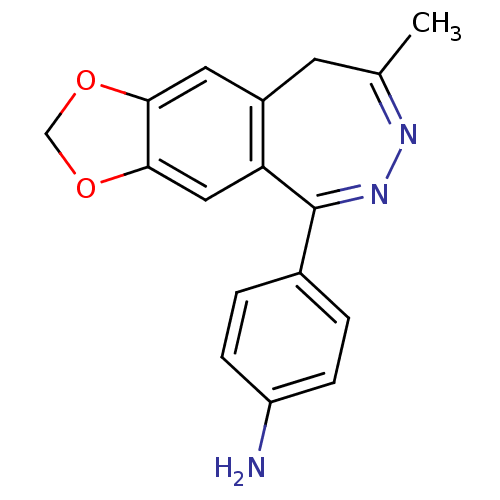
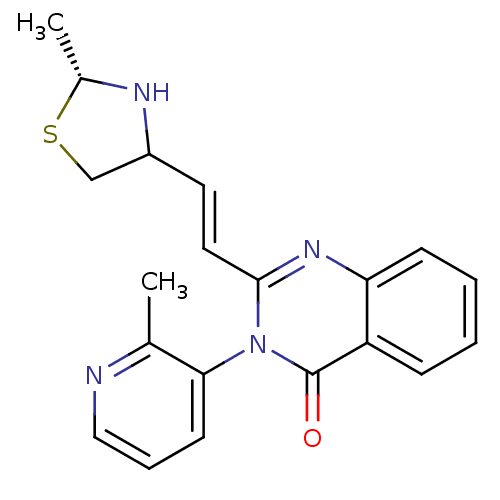
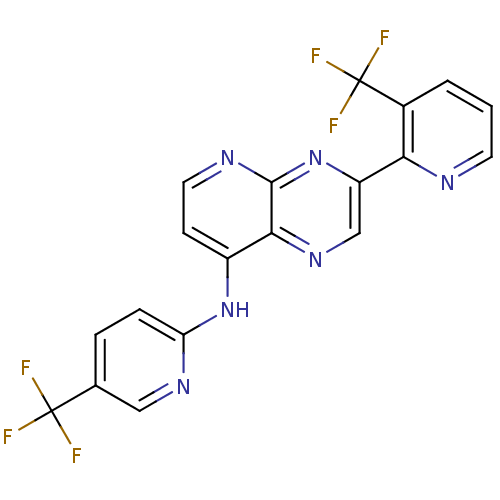
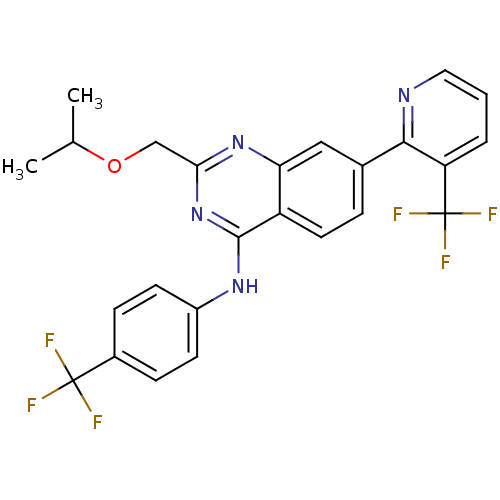
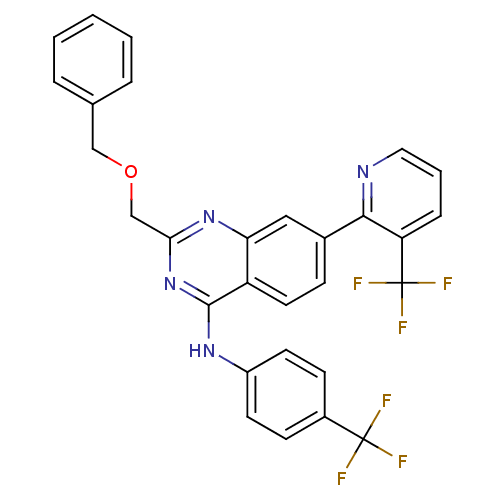
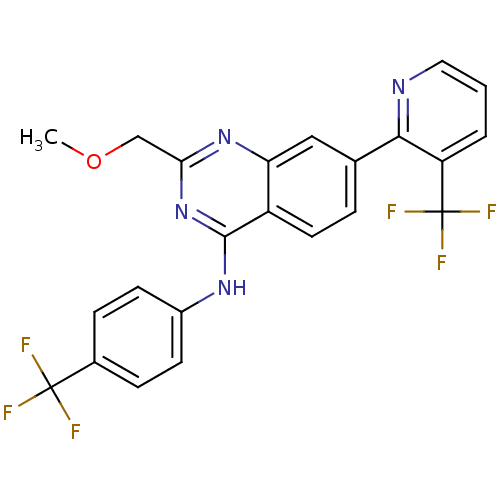
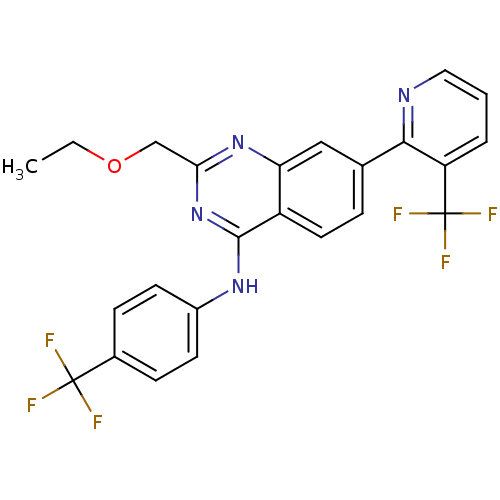
Affinity DataKi: 0.240nMAssay Description:Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 0.310nMAssay Description:Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 0.410nMAssay Description:Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 0.430nMAssay Description:Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 0.440nMAssay Description:Inhibition of human bradykinin B1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.590nMAssay Description:Inhibition of human bradykinin B1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.670nMAssay Description:Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 0.830nMAssay Description:Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 0.830nMAssay Description:Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 0.870nMAssay Description:Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 0.910nMAssay Description:Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 0.920nMAssay Description:Antagonist activity at rat bradykinin B1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 1.66nMAssay Description:Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 1.75nMAssay Description:Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 1.97nMAssay Description:Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 2.06nMAssay Description:Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 2.84nMAssay Description:Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 9.81nMAssay Description:Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 15.0nMAssay Description:Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 32.3nMAssay Description:Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 815nMAssay Description:Antagonist activity at human CB2 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+3nMAssay Description:Antagonist activity at rat bradykinin B1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.90E+3nMAssay Description:Antagonist activity at human CB2 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: 4.95E+3nMAssay Description:Antagonist activity at human CB2 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nMAssay Description:Antagonist activity at human CB2 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS bindingMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Neurogen
Curated by ChEMBL
Neurogen
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Antagonist activity at human TRPV1 assessed as inhibition of capsaicin-induced receptor activation by FLIPR assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Neurogen
Curated by ChEMBL
Neurogen
Curated by ChEMBL
Affinity DataIC50: 0.210nMAssay Description:Antagonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as inhibition of capsazepine-induced calcium mobilization by FLIPR ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Neurogen
Curated by ChEMBL
Neurogen
Curated by ChEMBL
Affinity DataIC50: 0.230nMAssay Description:Antagonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as inhibition of capsazepine-induced calcium mobilization by FLIPR ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Neurogen
Curated by ChEMBL
Neurogen
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activationMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Neurogen
Curated by ChEMBL
Neurogen
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activationMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Neurogen
Curated by ChEMBL
Neurogen
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activationMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Neurogen
Curated by ChEMBL
Neurogen
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activationMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rattus norvegicus (rat))
Neurogen
Curated by ChEMBL
Neurogen
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Antagonist activity at rat TRPV1 receptor assessed as inhibition of low pH-induced activationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.540nMAssay Description:Inverse agonist at human CB1 receptor expressed in SF9 cells assessed as decrease in GTPgammaS levelMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Neurogen
Curated by ChEMBL
Neurogen
Curated by ChEMBL
Affinity DataIC50: 0.630nMAssay Description:Antagonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as inhibition of capsazepine-induced calcium mobilization by FLIPR ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Antagonist activity at rat bradykinin B1 receptorMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Neurogen
Curated by ChEMBL
Neurogen
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Antagonist activity at human TRPV1 assessed as inhibition of capsaicin-induced receptor activation by FLIPR assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Neurogen
Curated by ChEMBL
Neurogen
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activationMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Neurogen
Curated by ChEMBL
Neurogen
Curated by ChEMBL
Affinity DataIC50: 0.830nMAssay Description:Antagonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as inhibition of capsazepine-induced calcium mobilization by FLIPR ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Neurogen
Curated by ChEMBL
Neurogen
Curated by ChEMBL
Affinity DataIC50: 0.850nMAssay Description:Antagonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as inhibition of capsazepine-induced calcium mobilization by FLIPR ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Neurogen
Curated by ChEMBL
Neurogen
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Antagonist activity at human TRPV1 receptor assessed as inhibition of capsaicin-induced activationMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Neurogen
Curated by ChEMBL
Neurogen
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibition of human TRPV1More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Hydra Biosciences
US Patent
Hydra Biosciences
US Patent
Affinity DataIC50: 1nMAssay Description:Compounds of Formula (I) inhibit the TRPA1 channel, as shown by measuring the in vitro inhibition of human TRPA1, provided in data tables shown in Ta...More data for this Ligand-Target Pair