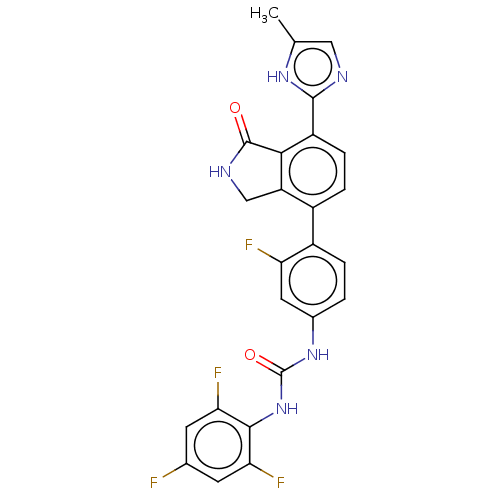
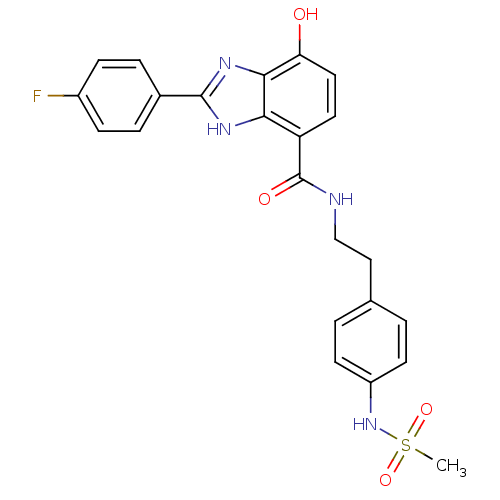
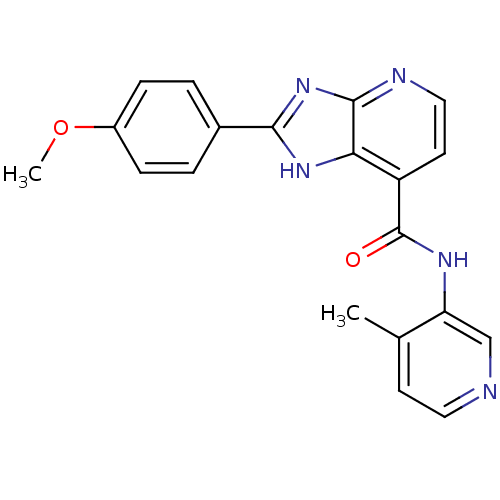
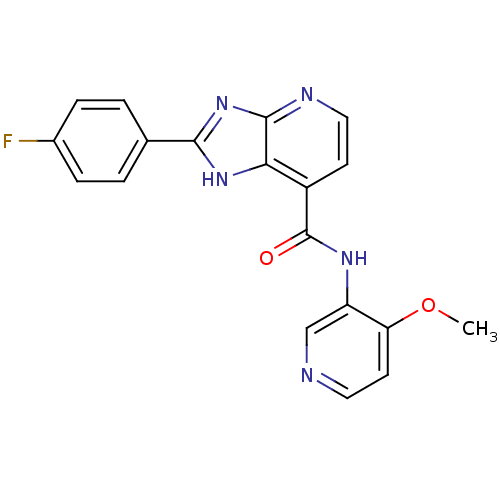
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
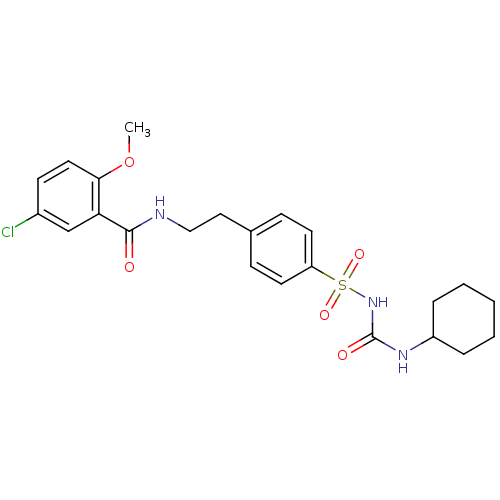
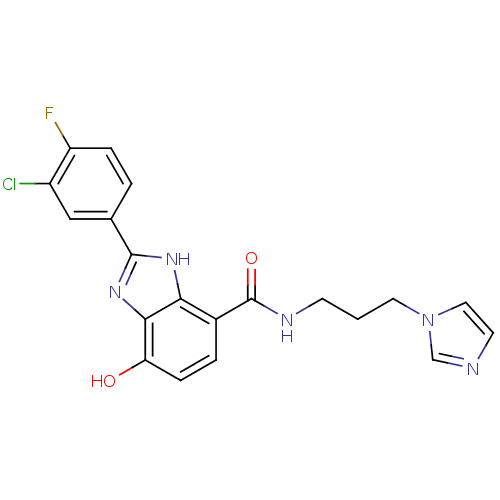
Affinity DataKi: 62nMAssay Description:Binding affinity to PPARgamma (unknown origin) by TR-FRET assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
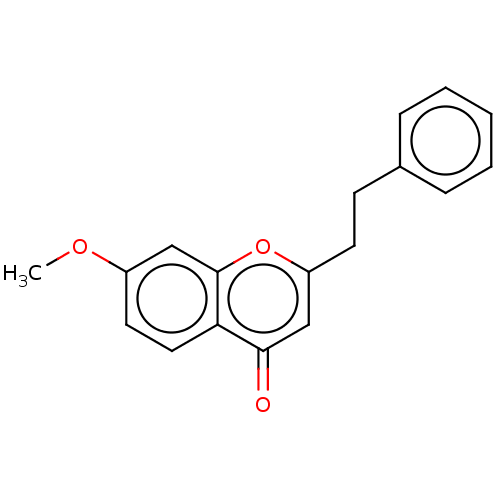
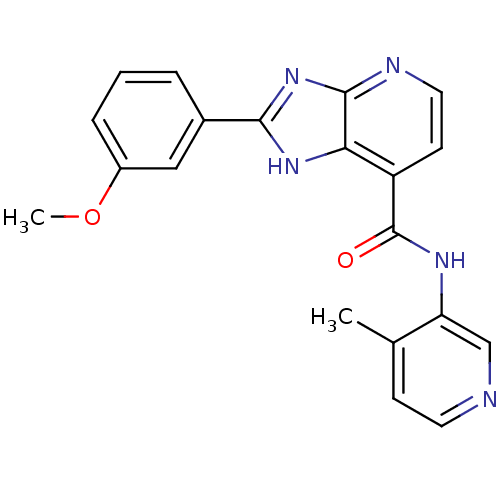
Affinity DataKi: 660nMAssay Description:Binding affinity to PPARgamma (unknown origin) by TR-FRET assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
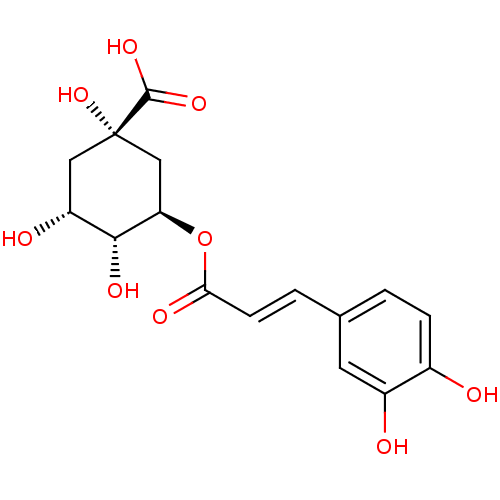
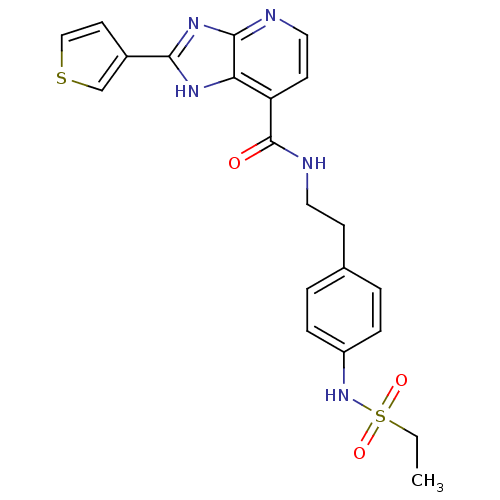
Affinity DataKi: 1.49E+4nMAssay Description:Binding affinity to PPARgamma (unknown origin) by TR-FRET assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
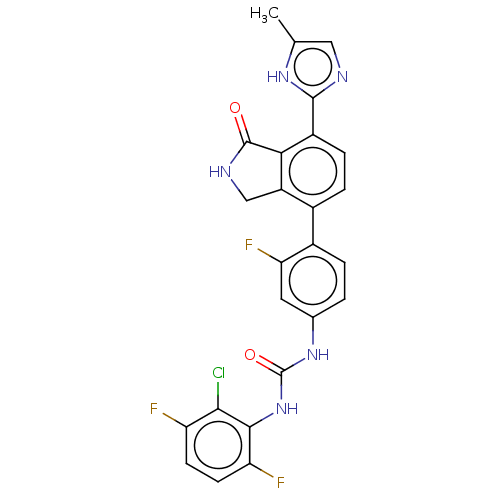
Affinity DataKi: 1.81E+4nMAssay Description:Binding affinity to PPARgamma (unknown origin) by TR-FRET assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataKi: 5.40E+4nMAssay Description:Binding affinity to PPARgamma (unknown origin) by TR-FRET assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1 [1-301](Homo sapiens (Human))
Washington College
Washington College
Affinity DataKi: 1.40E+6nMAssay Description:Steady-state kinetic parameters were measured for PTP1B using p-nitrophenyl phosphate (pNPP, 0.5−40 mM) as the substrate in NMR buffer. The rat...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1 [1-301](Homo sapiens (Human))
Washington College
Washington College
Affinity DataKi: 8.20E+6nMAssay Description:Steady-state kinetic parameters were measured for PTP1B using p-nitrophenyl phosphate (pNPP, 0.5−40 mM) as the substrate in NMR buffer. The rat...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0500nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0500nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0500nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0700nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0700nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0700nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.280nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.280nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.280nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of GSK3-betaMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human GSK3beta using phospho-CREB as substrate by beta counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human GSK3beta using phospho-CREB as substrate by beta counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human GSK3beta using phospho-CREB as substrate by beta counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human GSK3beta using phospho-CREB as substrate by beta counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human GSK3beta using phospho-CREB as substrate by beta counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human GSK3beta using phospho-CREB as substrate by beta counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human GSK3beta using phospho-CREB as substrate by beta counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human GSK3beta using phospho-CREB as substrate by beta counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human GSK3beta using phospho-CREB as substrate by beta counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human GSK3beta using phospho-CREB as substrate by beta counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of GSK3-betaMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human GSK3beta using phospho-CREB as substrate by beta counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human GSK3beta using phospho-CREB as substrate by beta counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of human GSK3beta using phospho-CREB as substrate by beta counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)