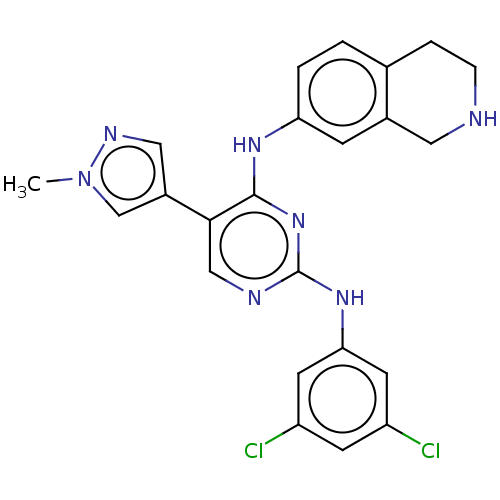
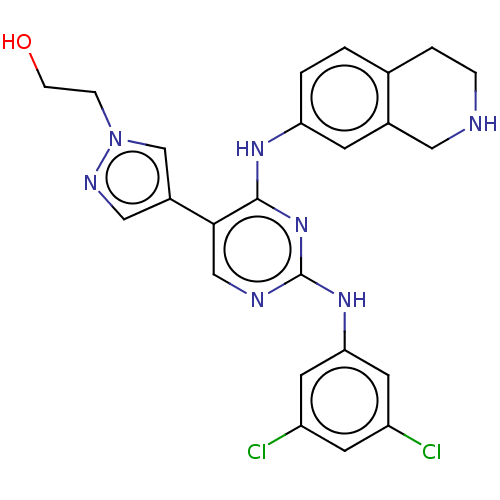
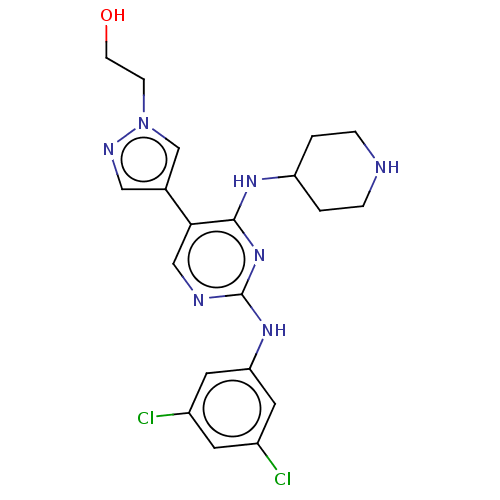
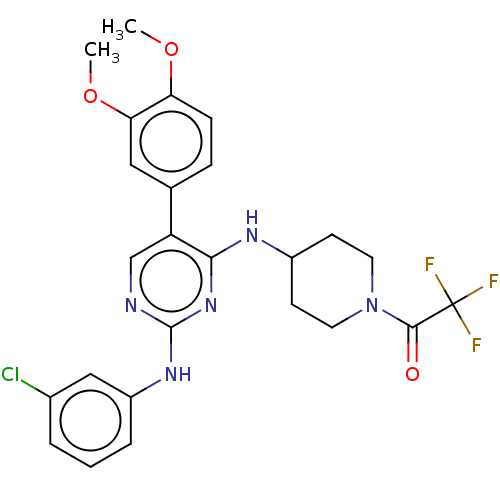
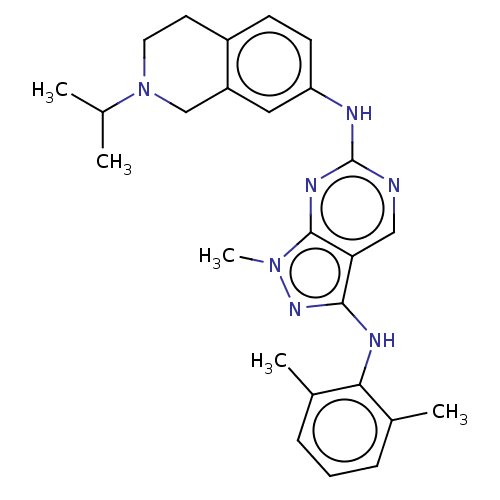
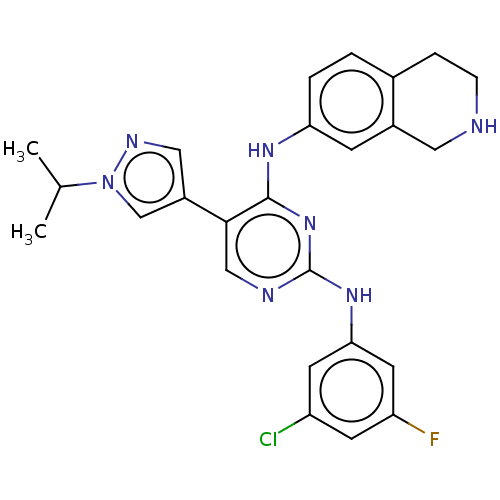
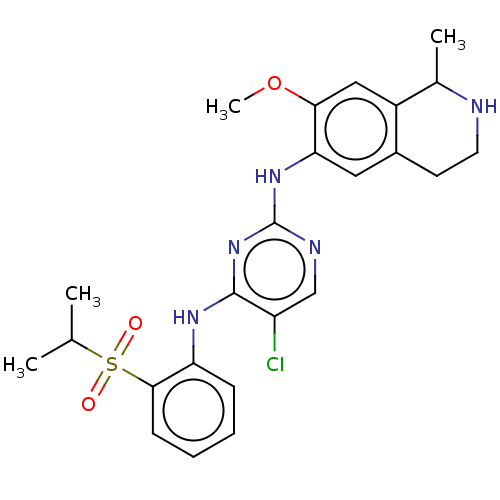
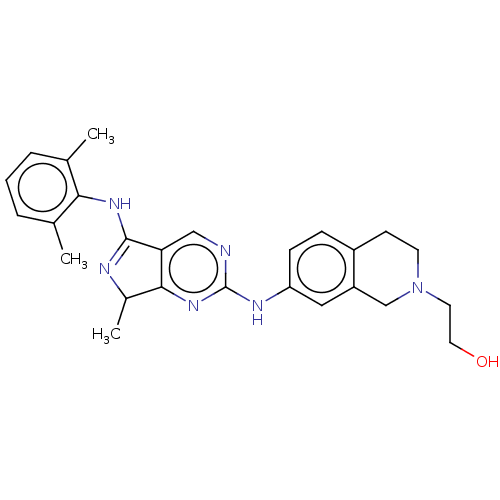
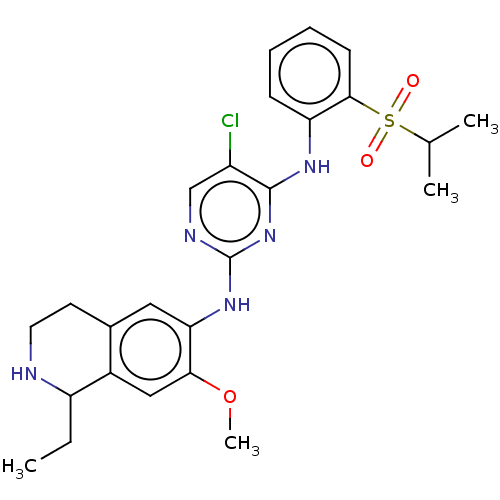
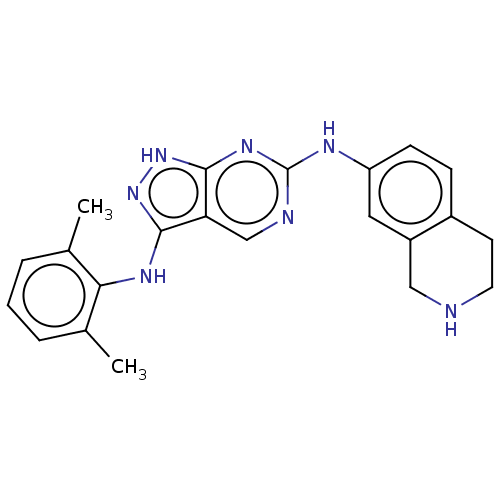
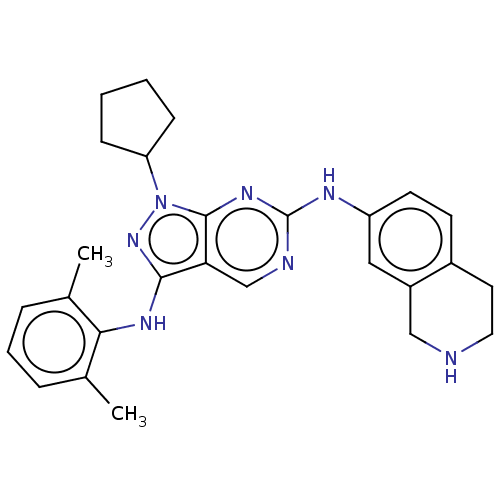
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.00600nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.0100nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.0120nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.0120nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.0120nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.0120nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.0410nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.0410nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.0520nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.0600nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.0970nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.100nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.120nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.130nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.130nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.130nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.140nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.140nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.160nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.160nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.160nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.170nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.170nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.170nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.190nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Korea Research Institute Of Chemical Technology
Curated by ChEMBL
Korea Research Institute Of Chemical Technology
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of recombinant c-Met by time resolved-fluorescence resonance energy transfer analysisMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.200nMAssay Description:BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.200nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.210nMT: 2°CAssay Description:A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor UFO(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.210nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.230nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.240nMAssay Description:Inhibition of crizotinib-resistant ALK G1269A mutant (unknown origin) using peptide substrate incubated for 30 mins in presence of ATP by fluorescenc...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.240nMT: 2°CAssay Description:A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.270nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.270nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.280nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.300nMAssay Description:BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.300nMAssay Description:BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.300nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Korea Research Institute Of Chemical Technology
Curated by ChEMBL
Korea Research Institute Of Chemical Technology
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of recombinant VEGFR-2 kinase domain by homogeneous time-resolved fluorescence assayMore data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor [L1196M](Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.300nMT: 2°CAssay Description:A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.300nMAssay Description:BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.300nMAssay Description:BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.320nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.340nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.340nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.360nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Korea Research Institute of Chemical Technology
US Patent
Korea Research Institute of Chemical Technology
US Patent
Affinity DataIC50: 0.370nMAssay Description:For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o...More data for this Ligand-Target Pair