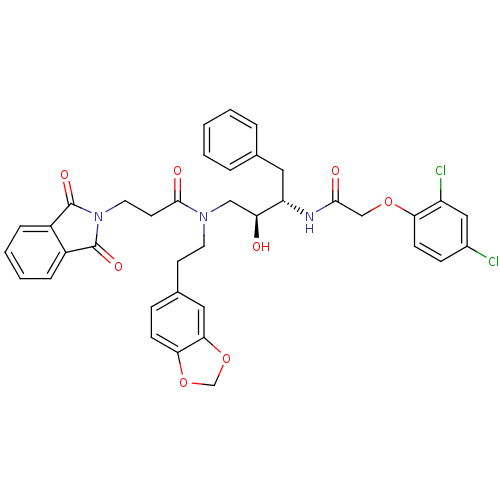
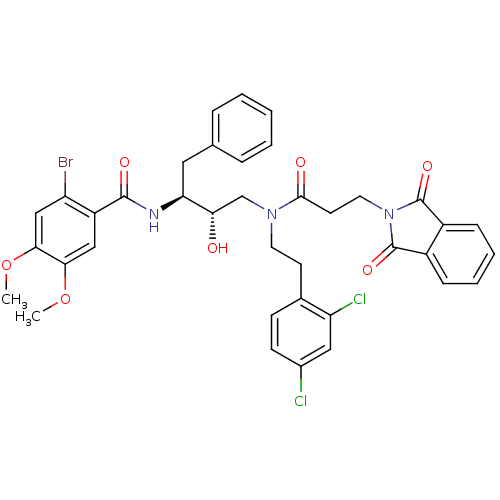
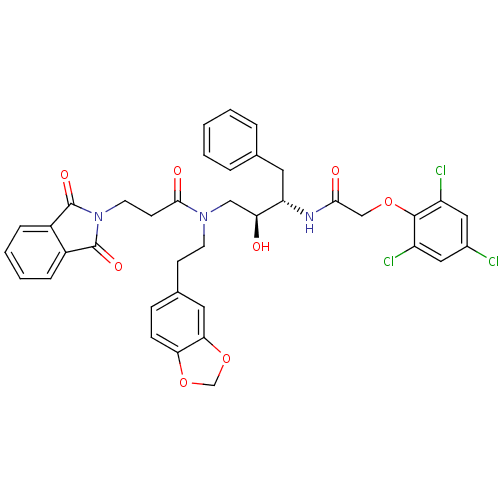
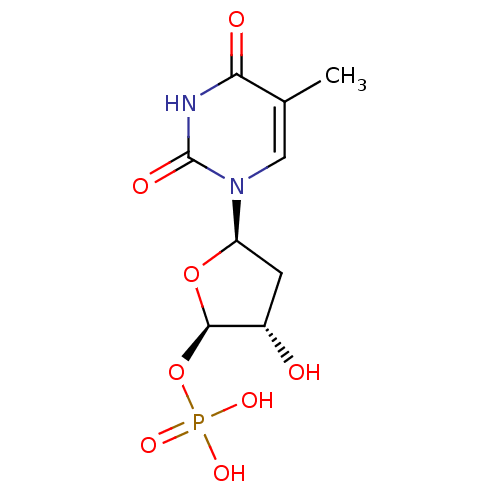
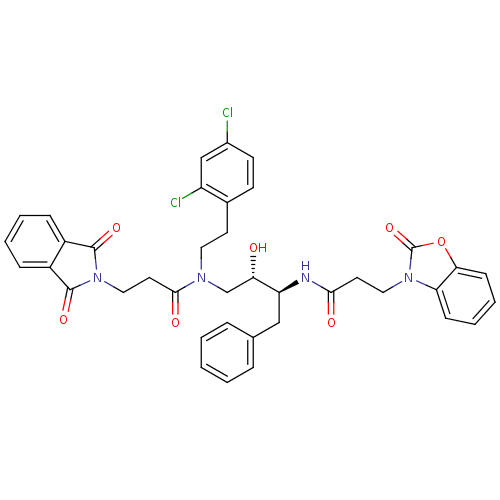
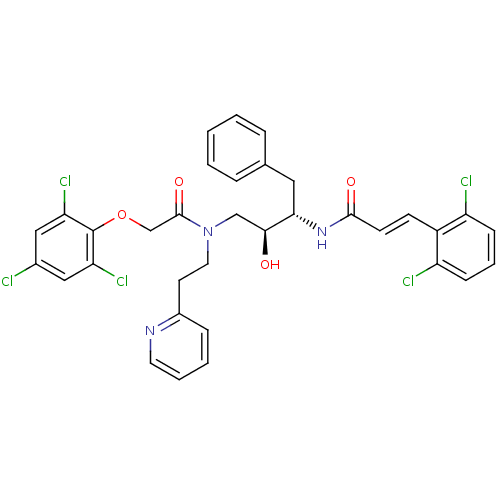
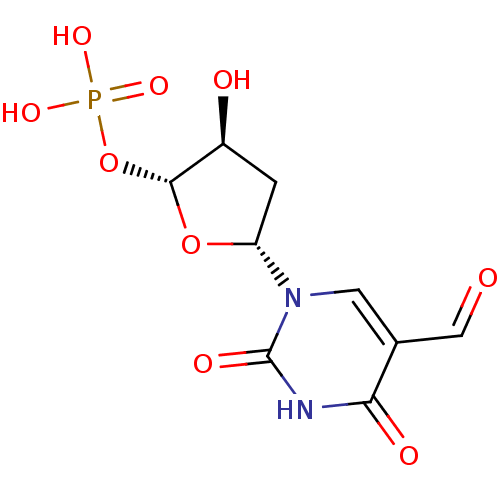
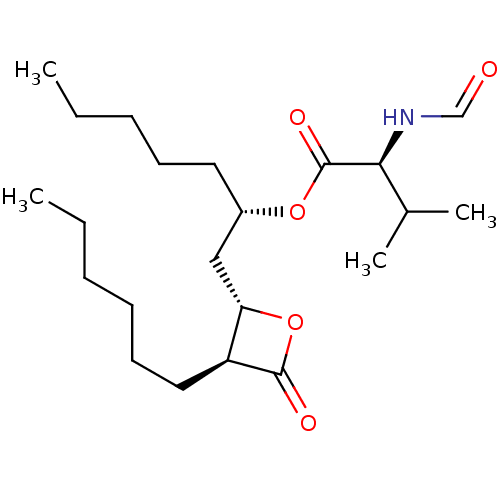
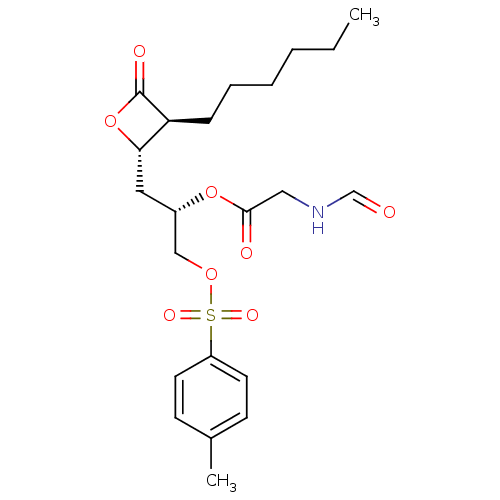
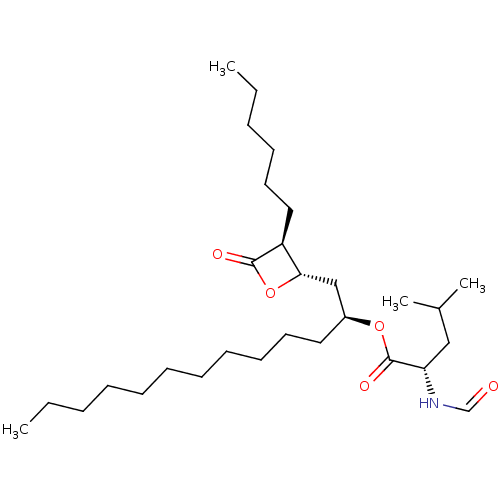
Affinity DataKi: 2.57nMAssay Description:Inhibition of mouse thymidylate synthase in mouse L1210 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Inhibitory activity of compound against human Cathepsin DMore data for this Ligand-Target Pair
Affinity DataKi: 5.20nMAssay Description:Inhibitory activity of compound against human Cathepsin DMore data for this Ligand-Target Pair
Affinity DataKi: 8.12nMAssay Description:Inhibition of mouse thymidylate synthase in mouse L1210 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 28.1nMAssay Description:Inhibition of mouse thymidylate synthase in mouse L1210 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 73nMAssay Description:Inhibitory activity of compound against human Cathepsin DMore data for this Ligand-Target Pair
Affinity DataKi: 128nMAssay Description:Inhibition of mouse thymidylate synthase in mouse L1210 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 138nMAssay Description:Inhibition of mouse thymidylate synthase in mouse L1210 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 231nMAssay Description:Inhibitory activity of compound against human Cathepsin DMore data for this Ligand-Target Pair
Affinity DataKi: 269nMAssay Description:Inhibition of mouse thymidylate synthase in mouse L1210 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 309nMAssay Description:Inhibition of mouse thymidylate synthase in mouse L1210 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 660nMAssay Description:Inhibition of mouse thymidylate synthase in mouse L1210 cellsMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:Inhibitory activity of compound against human Cathepsin DMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibitory activity of compound against human Cathepsin DMore data for this Ligand-Target Pair
Affinity DataKi: 1.62E+7nMAssay Description:Inhibition of mouse thymidylate synthase in mouse L1210 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.00E+7nMAssay Description:Inhibition of mouse thymidylate synthase in mouse L1210 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3.02E+8nMAssay Description:Inhibition of mouse thymidylate synthase in mouse L1210 cellsMore data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 100nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 120nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 180nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 210nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 230nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 280nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 290nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 300nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 370nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 400nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 450nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 500nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 500nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 520nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 680nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 790nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 900nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 1.02E+3nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 1.04E+3nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 1.35E+3nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 1.35E+3nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 1.72E+3nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 3.00E+3nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 3.67E+3nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 6.24E+3nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 9.74E+3nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 1.08E+4nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 1.35E+4nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair
TargetFatty acid synthase [2202-2509](Homo sapiens (Human))
The Burnham Institute For Medical Research
The Burnham Institute For Medical Research
Affinity DataIC50: 4.51E+4nMpH: 7.4 T: 2°CAssay Description:The reaction mixture consisted of FAS thioesterase domain (FASTE) in buffer, which was preincubated with test compounds for 30 min. The reaction was ...More data for this Ligand-Target Pair