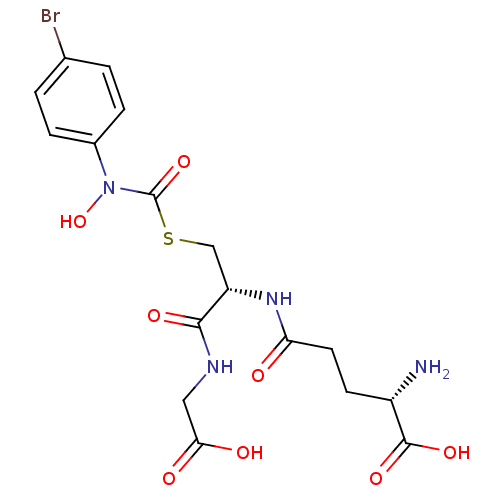
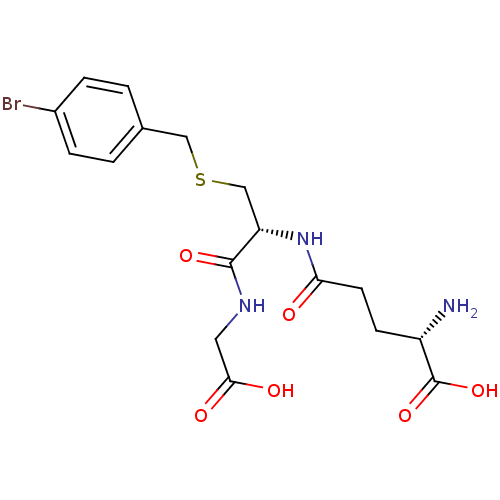
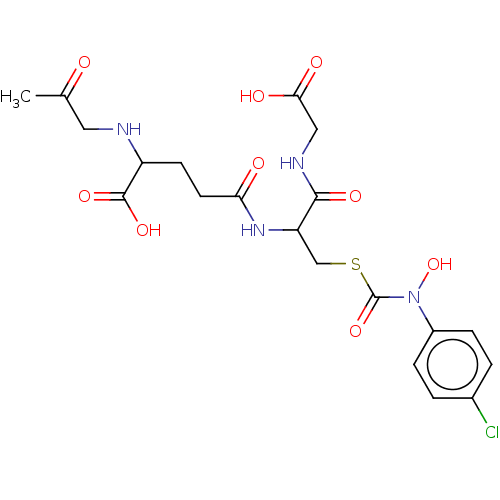
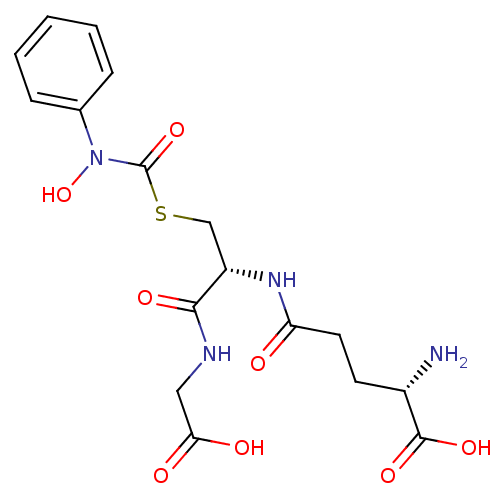
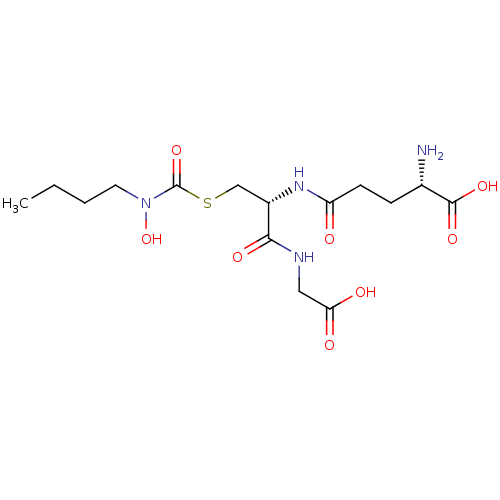
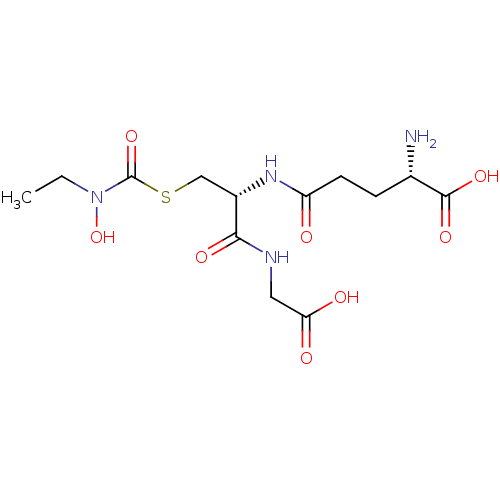
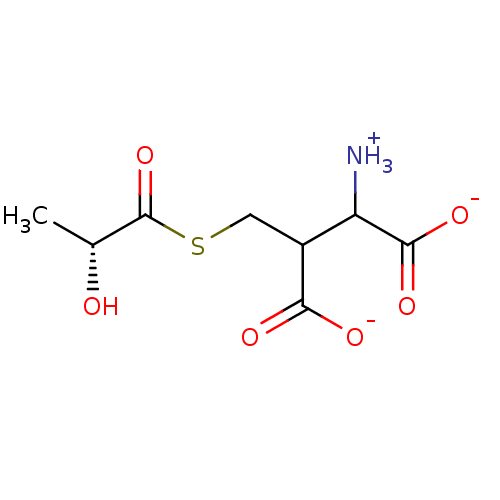
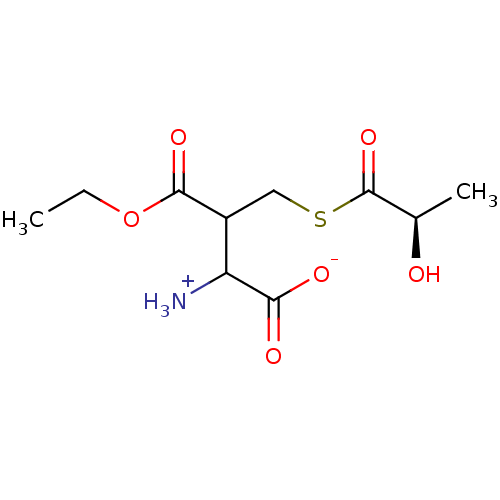
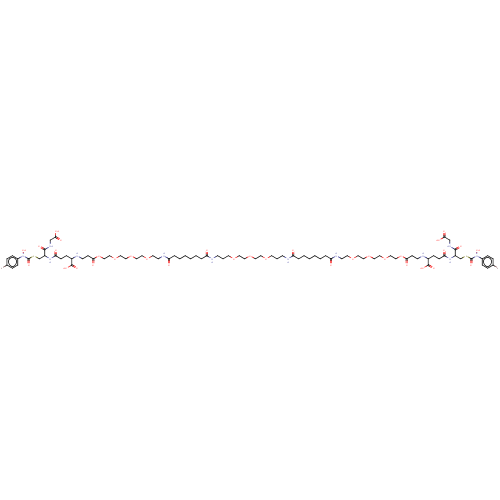Affinity DataKi: 0.300nMAssay Description:Inhibition of 6-His tagged recombinant human glyoxalase 1 transfected in Escherichia coli BL21 (DE3) assessed as S-D-lactoylglutathione formation by ...More data for this Ligand-Target Pair
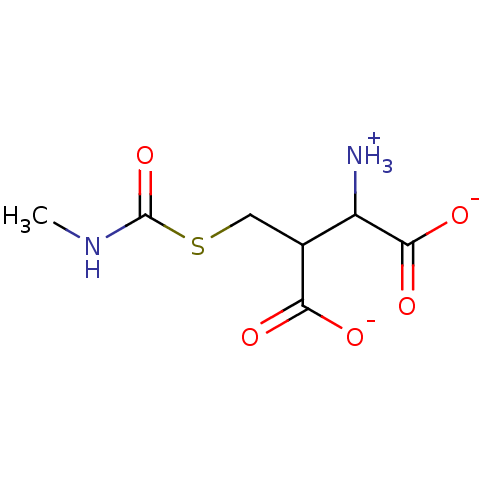
Affinity DataKi: 1nMAssay Description:Inhibition of 6-His tagged recombinant human glyoxalase 1 transfected in Escherichia coli BL21 (DE3) assessed as S-D-lactoylglutathione formation by ...More data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Tested for inhibitory activity against human erythrocyte glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Binding affinity for Glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Binding affinity for Glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Binding affinity for Glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 46nMAssay Description:Competitive inhibition of GLO1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 46nMAssay Description:Tested for inhibitory activity against human erythrocyte glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 46nMAssay Description:Binding affinity for Glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 46nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 46nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Inhibition of GLO1 (unknown origin)More data for this Ligand-Target Pair
TargetHydroxyacylglutathione hydrolase, mitochondrial(Bos taurus)
Chengdu University
Curated by ChEMBL
Chengdu University
Curated by ChEMBL
Affinity DataKi: 81nMAssay Description:Inhibition of bovine liver glyoxalase 2More data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
TargetHydroxyacylglutathione hydrolase, mitochondrial(Bos taurus)
Chengdu University
Curated by ChEMBL
Chengdu University
Curated by ChEMBL
Affinity DataKi: 138nMAssay Description:Inhibition of bovine liver glyoxalase 2More data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Tested for inhibitory activity against human erythrocyte glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Binding affinity for Glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 170nMAssay Description:Binding affinity for Glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 180nMAssay Description:Binding affinity for Glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 330nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
Affinity DataKi: 800nMAssay Description:Binding affinity for Glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 1.18E+3nMAssay Description:Binding affinity for Glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nMAssay Description:Binding affinity of the compound on yeast glyoxalase I (GlxI) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nMAssay Description:Tested for inhibitory activity against yeast glyoxalase IMore data for this Ligand-Target Pair
TargetHydroxyacylglutathione hydrolase, mitochondrial(Homo sapiens (Human))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataKi: 1.20E+3nMAssay Description:Inhibition constants obtained from using the enediol analogs as competitive inhibitors for the inhibition of thehydrolysis of S-D-lactoylglutathione ...More data for this Ligand-Target Pair
TargetHydroxyacylglutathione hydrolase, mitochondrial(Bos taurus)
Chengdu University
Curated by ChEMBL
Chengdu University
Curated by ChEMBL
Affinity DataKi: 1.70E+3nMAssay Description:Inhibition of bovine liver glyoxalase 2More data for this Ligand-Target Pair
Affinity DataKi: 1.70E+3nMAssay Description:Tested for inhibitory activity against human erythrocyte glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 1.70E+3nMAssay Description:Binding affinity for Glyoxalase IMore data for this Ligand-Target Pair
TargetHydroxyacylglutathione hydrolase, mitochondrial(Homo sapiens (Human))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataKi: 3.40E+3nMAssay Description:Inhibition constants obtained from using the enediol analogs as competitive inhibitors for the inhibition of thehydrolysis of S-D-lactoylglutathione ...More data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:Binding affinity of the compound on yeast glyoxalase I (GlxI) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:Tested for inhibitory activity against yeast glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMAssay Description:Binding affinity of the compound on Pseudomonas putida glyoxalase I (GlxI) was determinedMore data for this Ligand-Target Pair
TargetHydroxyacylglutathione hydrolase, mitochondrial(Homo sapiens (Human))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataKi: 1.10E+4nMAssay Description:Inhibition constant for the inhibition of the hydrolysis of S-D-lactoylglutathione by glyoxalase IIMore data for this Ligand-Target Pair
Affinity DataKi: 1.10E+4nMAssay Description:Tested for inhibitory activity against yeast glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 1.10E+4nMAssay Description:Binding affinity of the compound on yeast glyoxalase I (GlxI) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+4nMAssay Description:Binding affinity of the compound on Pseudomonas putida glyoxalase I (GlxI) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 2.80E+4nMAssay Description:Binding affinity of the compound on Pseudomonas putida glyoxalase I (GlxI) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 6.80E+4nMAssay Description:Tested for inhibitory activity against yeast glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 6.80E+4nMAssay Description:Binding affinity of the compound on yeast glyoxalase I (GlxI) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 8.60E+4nMAssay Description:Binding affinity of the compound on Pseudomonas putida glyoxalase I (GlxI) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 1.83E+5nMAssay Description:Binding affinity for Glyoxalase IMore data for this Ligand-Target Pair
TargetHydroxyacylglutathione hydrolase, mitochondrial(Homo sapiens (Human))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataKi: 4.26E+5nMAssay Description:Inhibition constants obtained from using the enediol analogs as competitive inhibitors for the inhibition of thehydrolysis of S-D-lactoylglutathione ...More data for this Ligand-Target Pair
Affinity DataKi: 6.80E+5nMAssay Description:Tested for inhibitory activity against yeast glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 1.98E+6nMAssay Description:Tested for inhibitory activity against yeast glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 2.23E+6nMAssay Description:Tested for inhibitory activity against yeast glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 3.16E+6nMAssay Description:Tested for inhibitory activity against yeast glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 5.67E+6nMAssay Description:Tested for inhibitory activity against yeast glyoxalase IMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibitory concentration against GSTP1-1 overexpressed in MCF-7piGST cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.08E+4nMAssay Description:Inhibitory concentration against GSTP1-1 overexpressed in MCF7wt cellsMore data for this Ligand-Target Pair