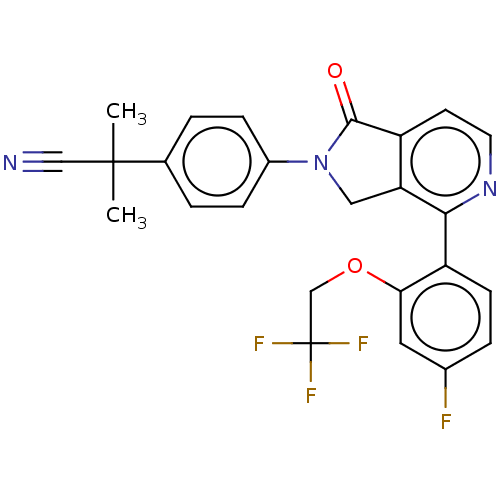
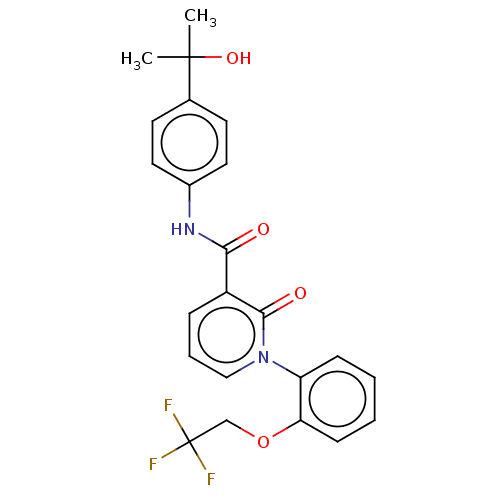
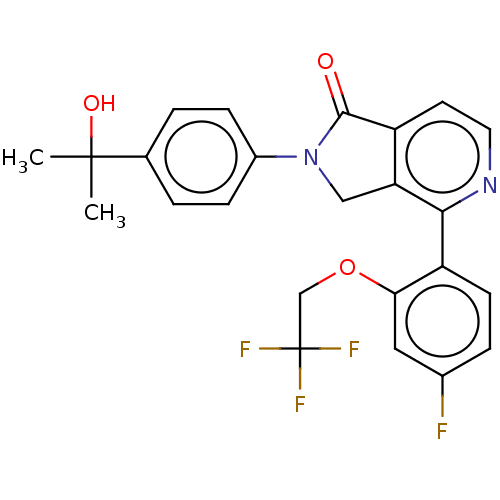
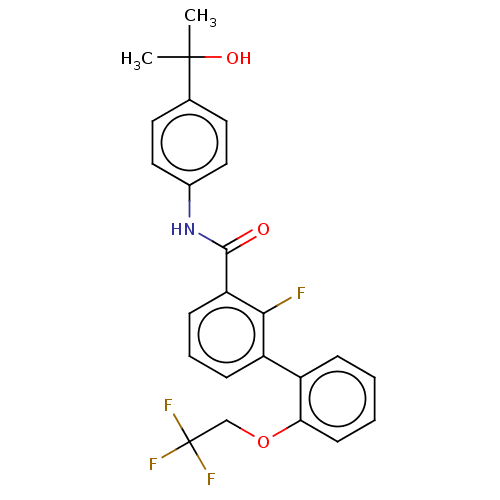
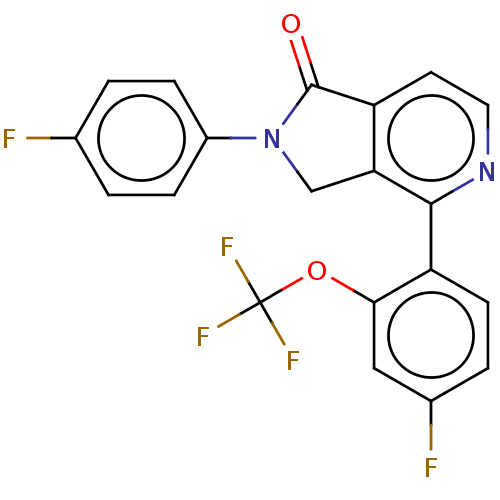
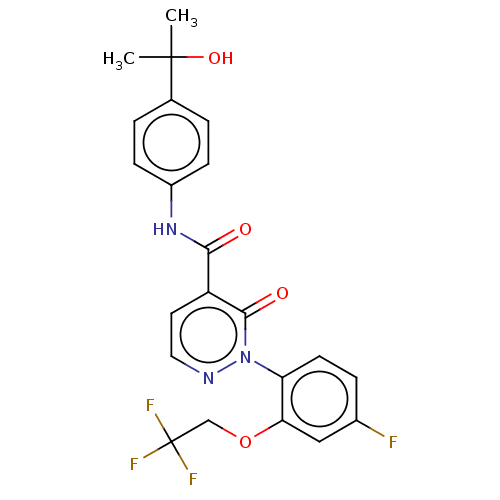
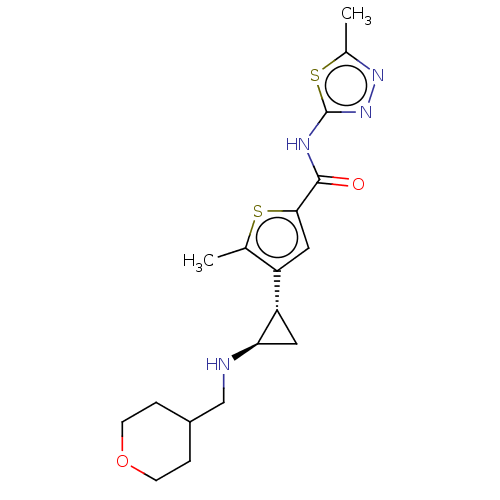
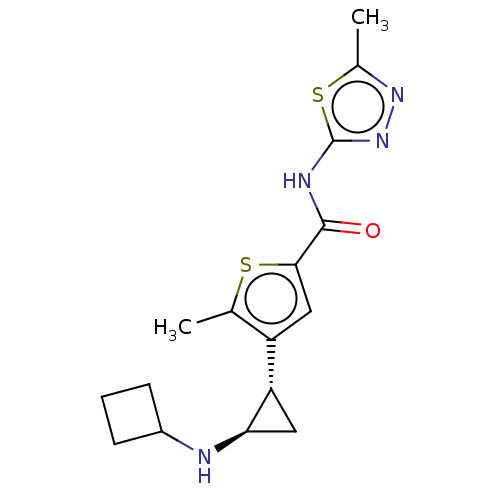
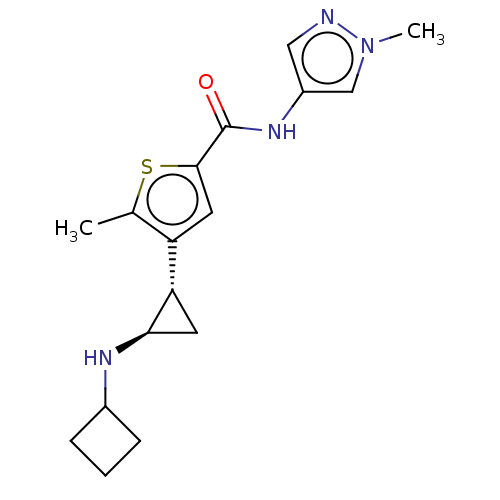
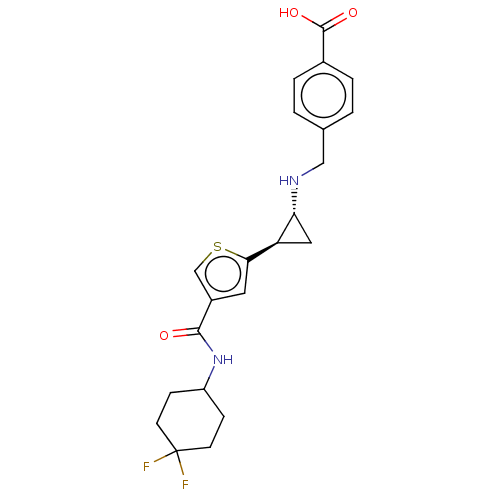
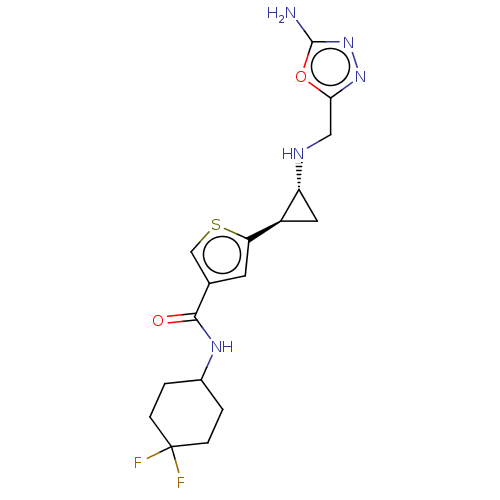
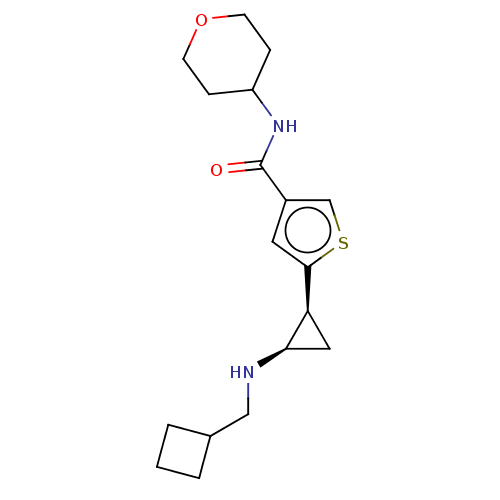
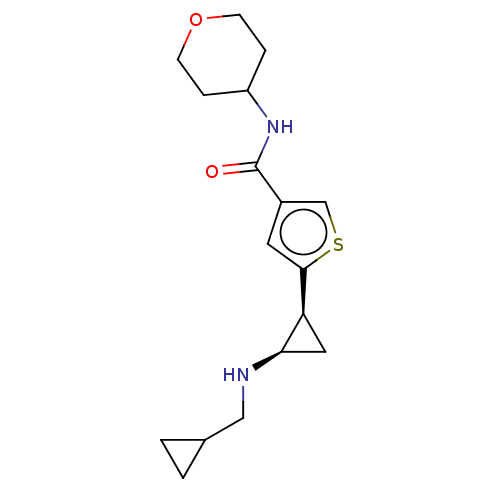
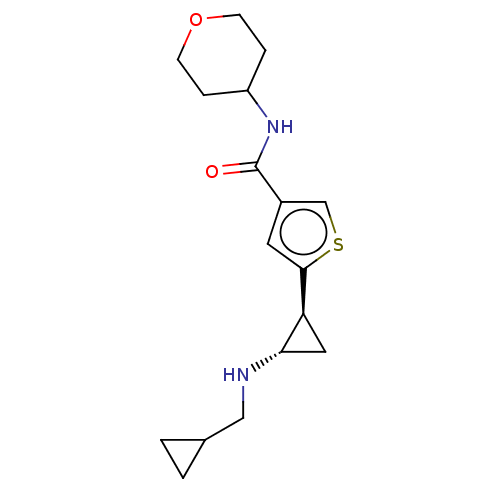
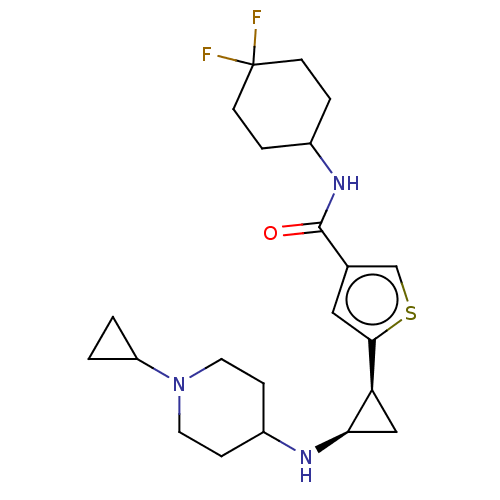
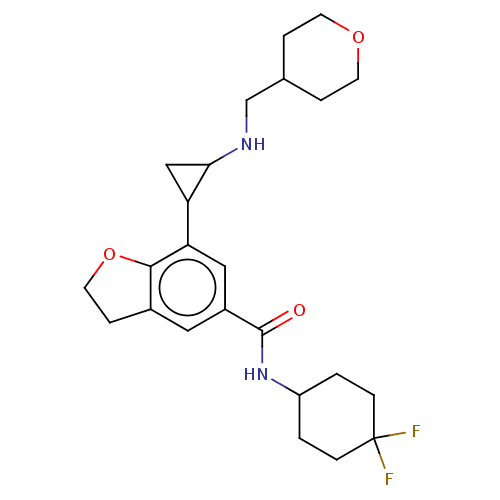
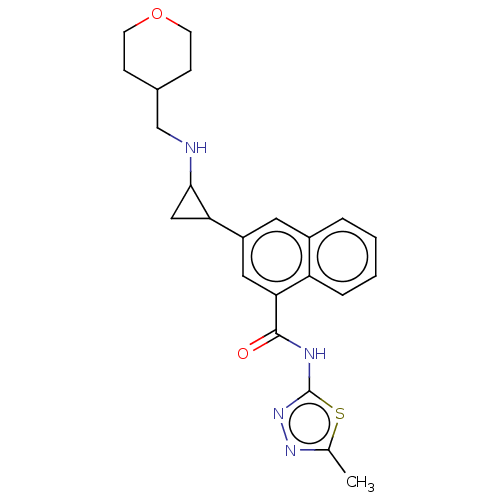
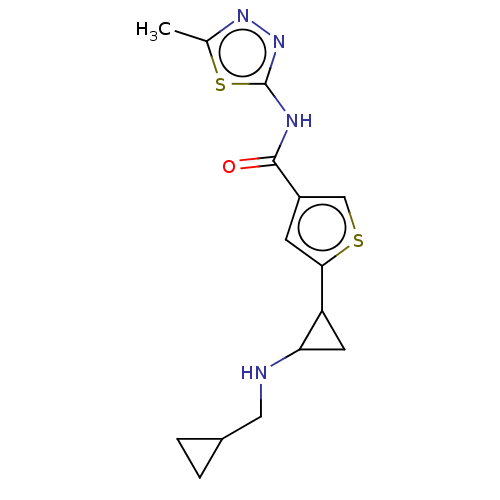
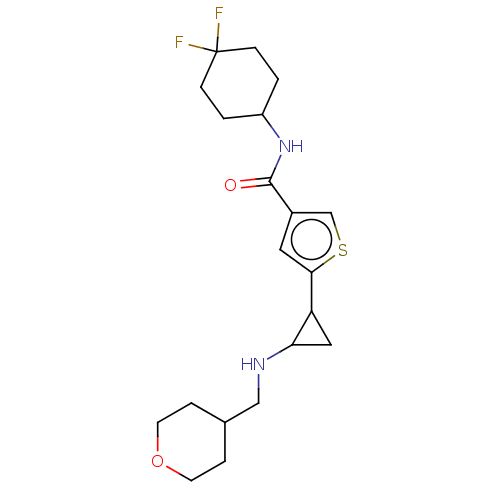
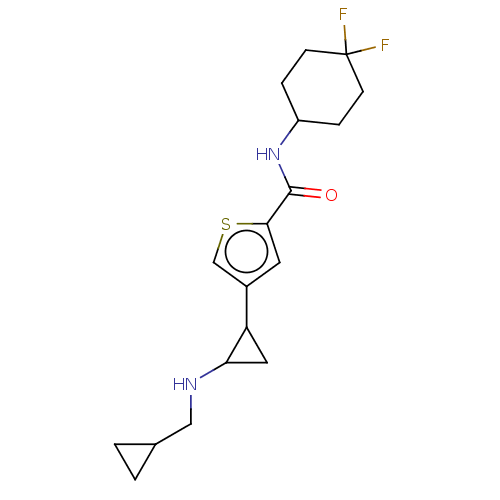
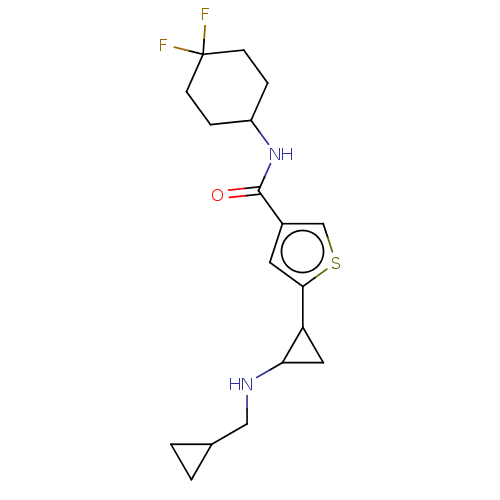
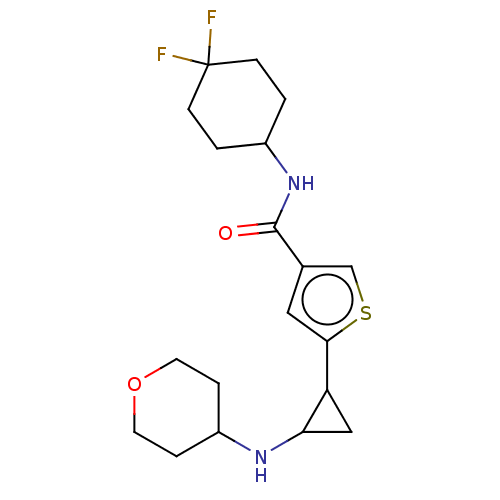
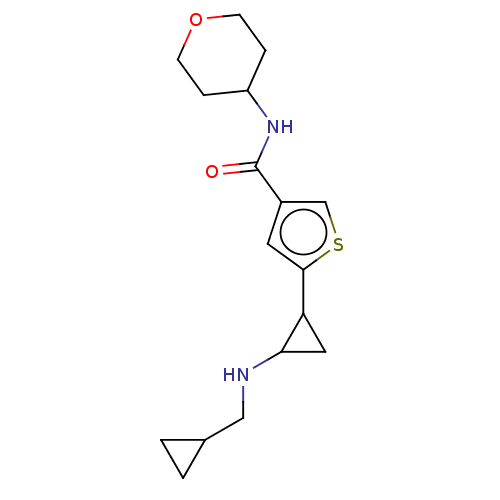
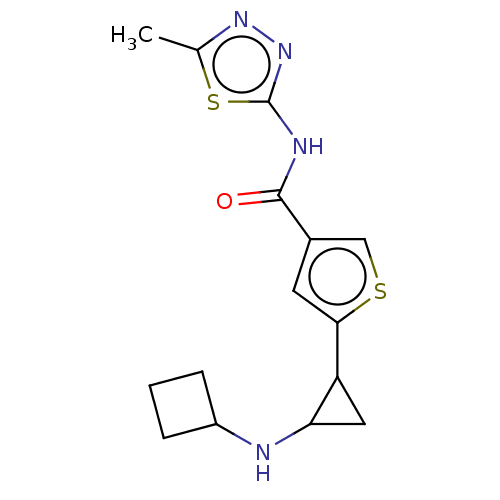
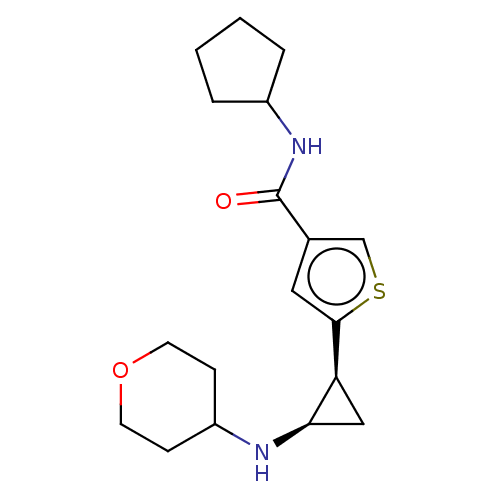
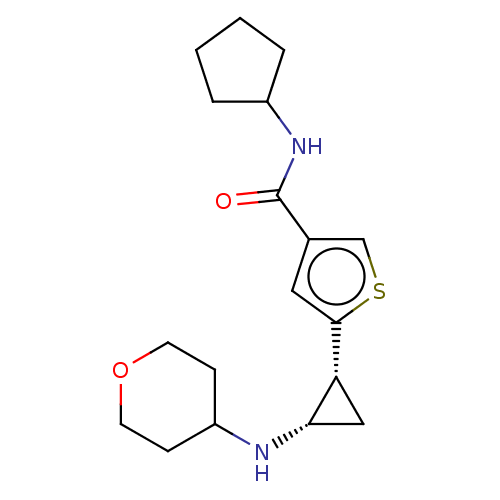
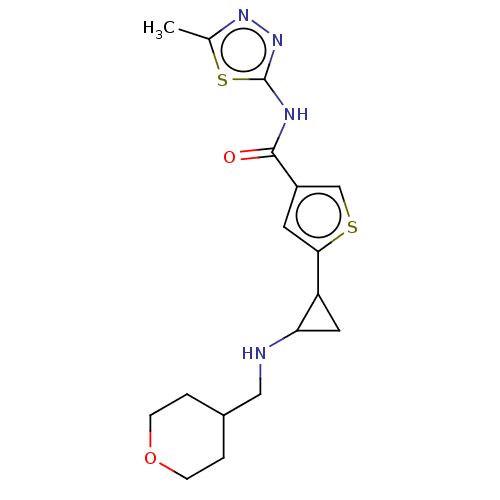
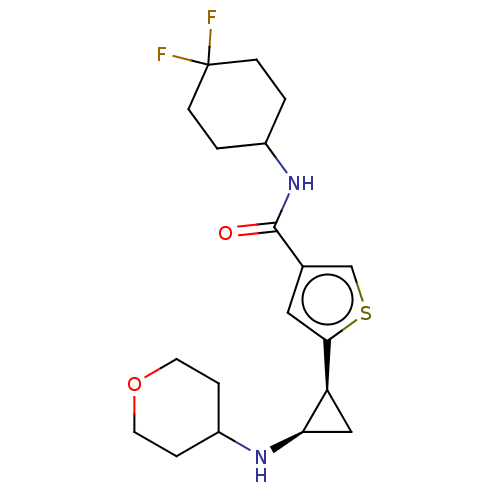
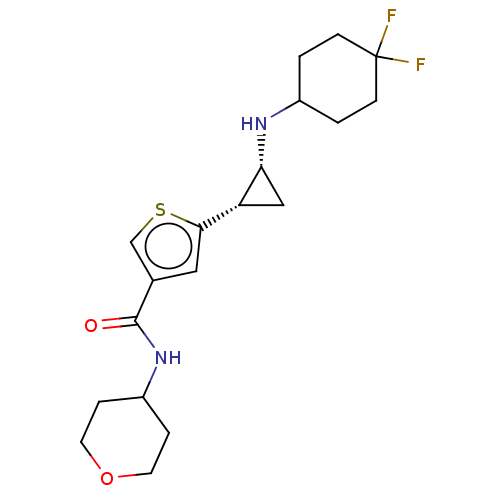
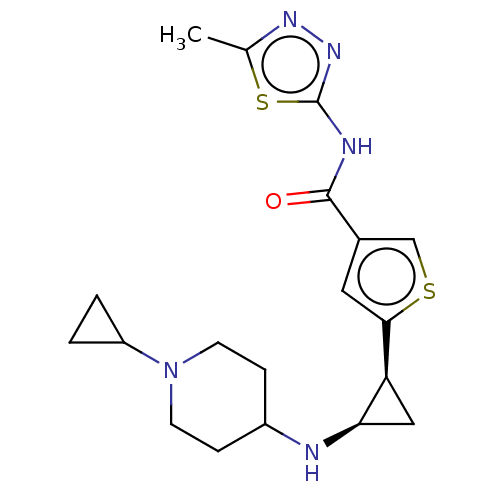
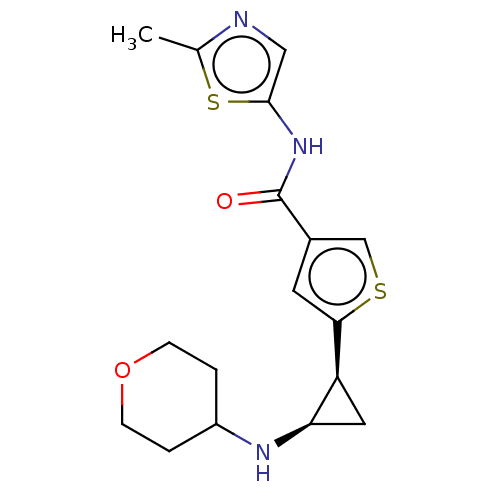
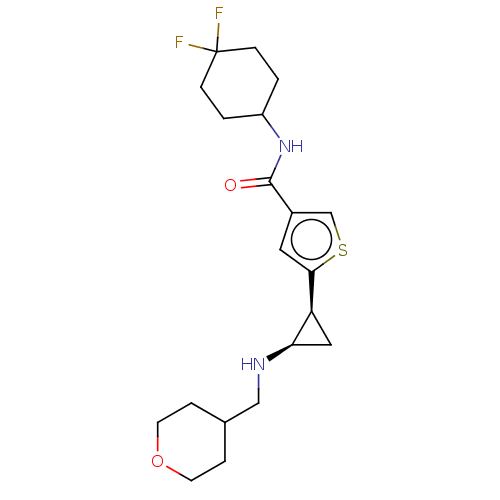
Affinity DataIC50: 15nMAssay Description:Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometryMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometryMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometryMore data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometryMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometryMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometryMore data for this Ligand-Target Pair
Affinity DataIC50: 49nMAssay Description:Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometryMore data for this Ligand-Target Pair
Affinity DataIC50: 49nMAssay Description:Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometryMore data for this Ligand-Target Pair
Affinity DataIC50: 51nMAssay Description:Inhibition of mouse GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometryMore data for this Ligand-Target Pair
Affinity DataIC50: 97nMAssay Description:Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: 100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: 100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833](Homo sapiens (Human))
Takeda Pharmaceutical
US Patent
Takeda Pharmaceutical
US Patent
Affinity DataIC50: <100nMpH: 8.0 T: 2°CAssay Description:A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m...More data for this Ligand-Target Pair