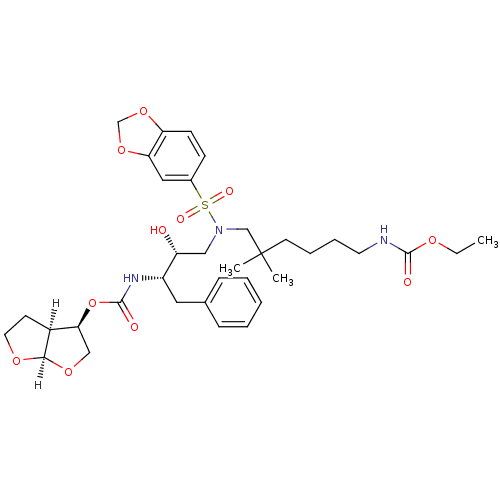
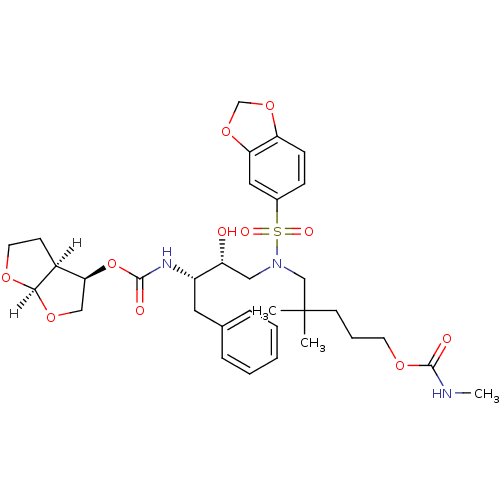
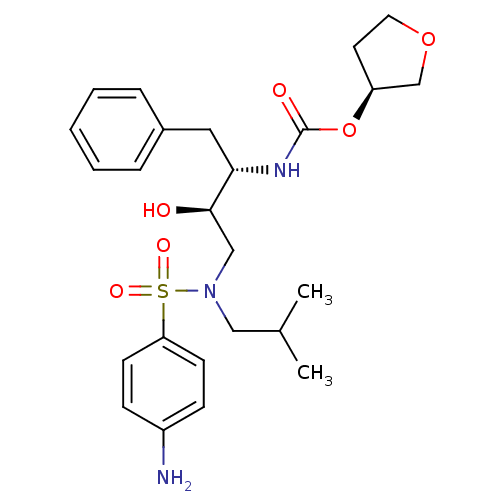
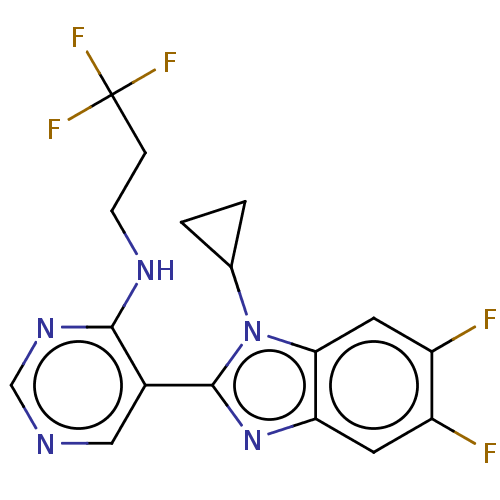
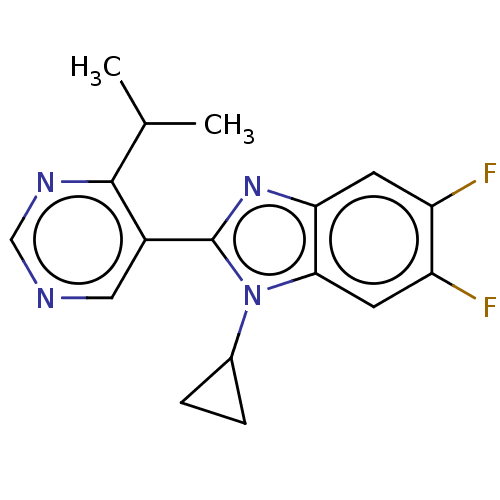
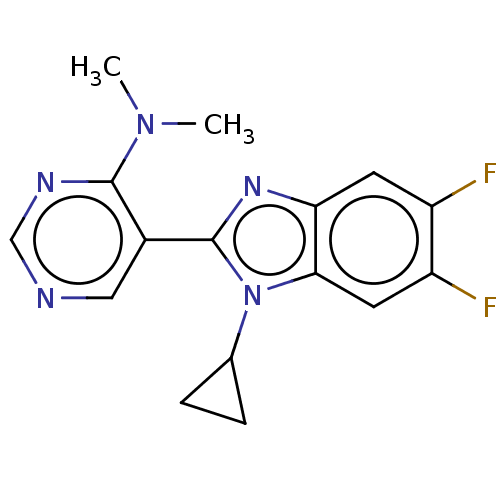
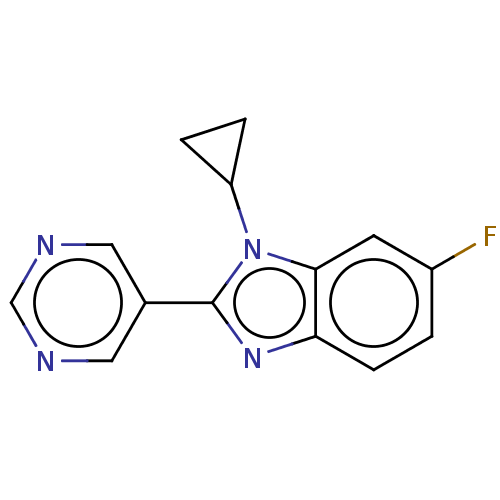
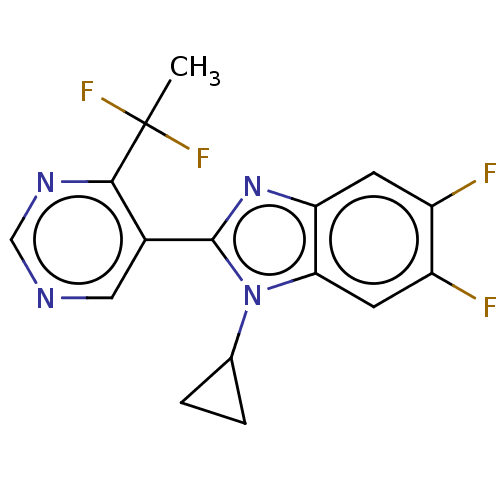
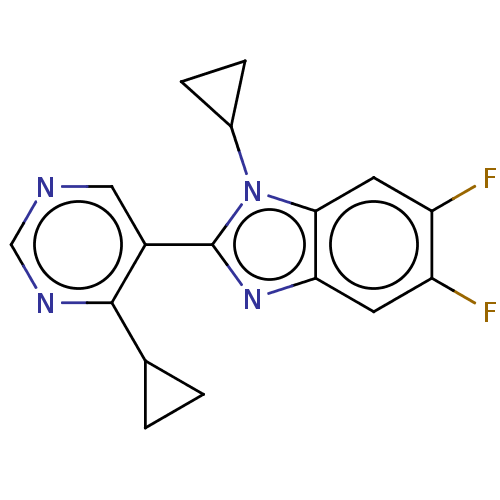
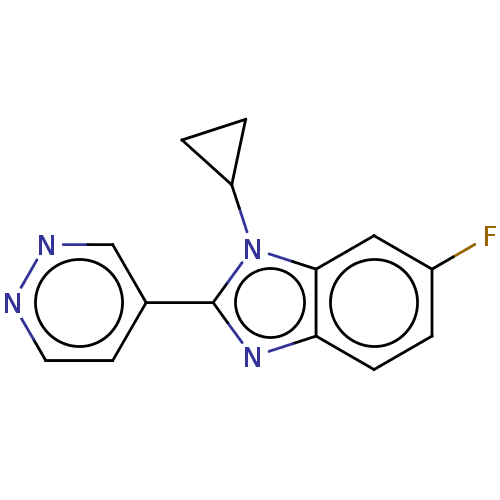
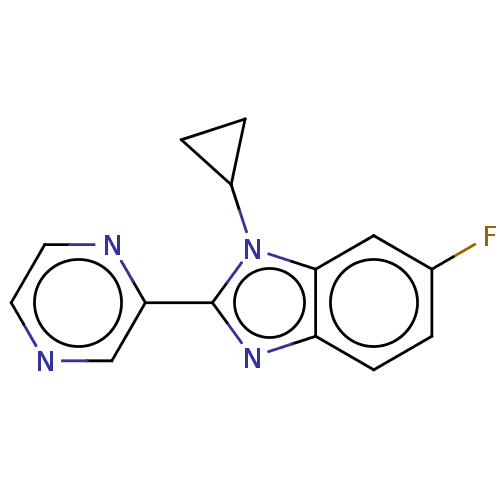
Affinity DataKi: 0.00000600nM ΔG°: -82.5kJ/molepH: 5.5 T: 2°CAssay Description:The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of...More data for this Ligand-Target Pair
Affinity DataKi: 0.0000130nM ΔG°: -80.6kJ/molepH: 5.5 T: 2°CAssay Description:The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of...More data for this Ligand-Target Pair
Affinity DataKi: 0.0000150nM ΔG°: -80.2kJ/molepH: 5.5 T: 2°CAssay Description:The assay method employed kinetic determinations of values for k1 and k-1, from which value of inhibition constant (Ki ) was determined (k-1/k1). The...More data for this Ligand-Target Pair
Affinity DataKi: 0.000165nM ΔG°: -74.2kJ/molepH: 5.5 T: 2°CAssay Description:The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,M535I,L552P,A560V,V571F,I573V](Human immunodeficiency virus type 1)
Glaxosmithkline
Glaxosmithkline
Affinity DataKi: 0.000220nM ΔG°: -73.5kJ/molepH: 5.5 T: 2°CAssay Description:Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of...More data for this Ligand-Target Pair
Affinity DataKi: 0.000240nM ΔG°: -73.2kJ/molepH: 5.5 T: 2°CAssay Description:The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,M535I,L552P,A560V,V571F,I573V](Human immunodeficiency virus type 1)
Glaxosmithkline
Glaxosmithkline
Affinity DataKi: 0.000420nM ΔG°: -71.8kJ/molepH: 5.5 T: 2°CAssay Description:Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,M535I,L552P,A560V,V571F,I573V](Human immunodeficiency virus type 1)
Glaxosmithkline
Glaxosmithkline
Affinity DataKi: 0.000750nM ΔG°: -70.4kJ/molepH: 5.5 T: 2°CAssay Description:The assay method employed kinetic determinations of values for k1 and k-1, from which value of inhibition constant (Ki ) was determined (k-1/k1). The...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,L499I,L508Q,K509R,E524D,M525I,S526N,M535I,I539V,I543V,I551V,L552P,A560V,V571A,L579M](Human immunodeficiency virus type 1)
Glaxosmithkline
Glaxosmithkline
Affinity DataKi: 0.00120nM ΔG°: -69.2kJ/molepH: 5.5 T: 2°CAssay Description:Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,L499I,L508Q,K509R,E524D,M525I,S526N,M535I,I539V,I543V,I551V,L552P,A560V,V571A,L579M](Human immunodeficiency virus type 1)
Glaxosmithkline
Glaxosmithkline
Affinity DataKi: 0.00170nM ΔG°: -68.3kJ/molepH: 5.5 T: 2°CAssay Description:Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,I539V](Human immunodeficiency virus type 1)
Glaxosmithkline
Glaxosmithkline
Affinity DataKi: 0.00200nM ΔG°: -67.9kJ/molepH: 5.5 T: 2°CAssay Description:The assay method employed kinetic determinations of values for k1 and k-1, from which value of inhibition constant (Ki ) was determined (k-1/k1). The...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,L499I,L508Q,K509R,E524D,M525I,S526N,M535I,I539V,I543V,I551V,L552P,A560V,V571A,L579M](Human immunodeficiency virus type 1)
Glaxosmithkline
Glaxosmithkline
Affinity DataKi: 0.00240nM ΔG°: -67.4kJ/molepH: 5.5 T: 2°CAssay Description:Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,I539V](Human immunodeficiency virus type 1)
Glaxosmithkline
Glaxosmithkline
Affinity DataKi: 0.00260nM ΔG°: -67.2kJ/molepH: 5.5 T: 2°CAssay Description:The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,L499I,L508Q,K509R,E524D,M525I,S526N,M535I,I539V,I543V,I551V,L552P,A560V,V571A,L579M](Human immunodeficiency virus type 1)
Glaxosmithkline
Glaxosmithkline
Affinity DataKi: 0.00340nM ΔG°: -66.6kJ/molepH: 5.5 T: 2°CAssay Description:The assay method employed kinetic determinations of values for k1 and k-1, from which value of inhibition constant (Ki ) was determined (k-1/k1). The...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,I539V](Human immunodeficiency virus type 1)
Glaxosmithkline
Glaxosmithkline
Affinity DataKi: 0.00390nM ΔG°: -66.2kJ/molepH: 5.5 T: 2°CAssay Description:The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,M535I,L552P,A560V,V571F,I573V](Human immunodeficiency virus type 1)
Glaxosmithkline
Glaxosmithkline
Affinity DataKi: 0.00430nM ΔG°: -66.0kJ/molepH: 5.5 T: 2°CAssay Description:Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,I539V](Human immunodeficiency virus type 1)
Glaxosmithkline
Glaxosmithkline
Affinity DataKi: 0.00460nM ΔG°: -65.8kJ/molepH: 5.5 T: 2°CAssay Description:The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,M535I,L552P,A560V,V571F,I573V](Human immunodeficiency virus type 1)
Glaxosmithkline
Glaxosmithkline
Affinity DataKi: 0.0250nM ΔG°: -61.5kJ/molepH: 5.5 T: 2°CAssay Description:Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,I539V](Human immunodeficiency virus type 1)
Glaxosmithkline
Glaxosmithkline
Affinity DataKi: 0.0270nM ΔG°: -61.3kJ/molepH: 5.5 T: 2°CAssay Description:The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,L499I,L508Q,K509R,E524D,M525I,S526N,M535I,I539V,I543V,I551V,L552P,A560V,V571A,L579M](Human immunodeficiency virus type 1)
Glaxosmithkline
Glaxosmithkline
Affinity DataKi: 0.0544nM ΔG°: -59.6kJ/molepH: 5.5 T: 2°CAssay Description:Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of...More data for this Ligand-Target Pair
Affinity DataKi: 0.0570nM ΔG°: -59.5kJ/molepH: 5.5 T: 2°CAssay Description:Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,M535I,L552P,A560V,V571F,I573V](Human immunodeficiency virus type 1)
Glaxosmithkline
Glaxosmithkline
Affinity DataKi: 2.30nM ΔG°: -50.1kJ/molepH: 5.5 T: 2°CAssay Description:Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,I539V](Human immunodeficiency virus type 1)
Glaxosmithkline
Glaxosmithkline
Affinity DataKi: 4.90nM ΔG°: -48.2kJ/molepH: 5.5 T: 2°CAssay Description:Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of...More data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587,L499I,L508Q,K509R,E524D,M525I,S526N,M535I,I539V,I543V,I551V,L552P,A560V,V571A,L579M](Human immunodeficiency virus type 1)
Glaxosmithkline
Glaxosmithkline
Affinity DataKi: 58nM ΔG°: -42.0kJ/molepH: 5.5 T: 2°CAssay Description:Enzymatic activity was determined with fluorogenic substrate in the presence and absence of inhibitor. The fluorescence increase due to hydrolysis of...More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: <2nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 7.90nMAssay Description:Inhibition of mouse FFA4 receptor expressed in U2OS cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 7.90nMAssay Description:Inhibition of rat FFA4 receptor expressed in U2OS cellsMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 24nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 29nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 29nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 62nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 79nMAssay Description:Inhibition of human FFA4 receptor expressed in U2OS cellsMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 172nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 330nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 11B1, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 378nMAssay Description:Inhibition of human recombinant CYP11B1 using deoxycortisol as substrate incubated for 60 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 11B1, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 390nMAssay Description:Inhibition of human recombinant CYP11B1 using deoxycortisol as substrate incubated for 60 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 412nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Selenity Therapeutics
Curated by ChEMBL
Selenity Therapeutics
Curated by ChEMBL
Affinity DataIC50: 541nMAssay Description:Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysisMore data for this Ligand-Target Pair


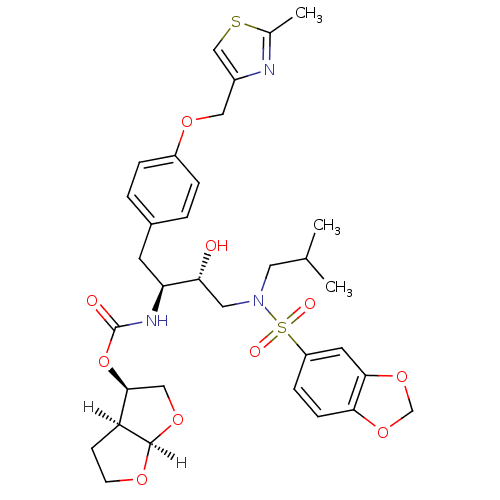
 3D Structure (crystal)
3D Structure (crystal)