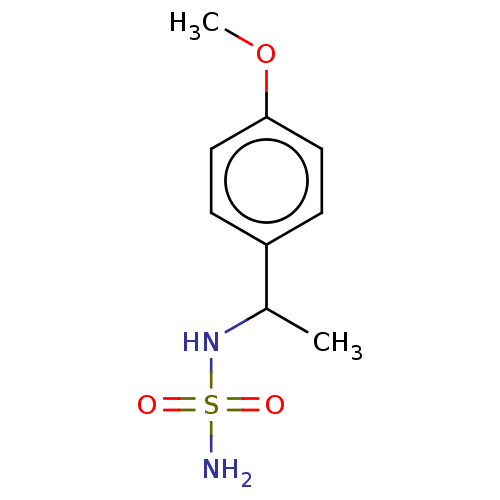
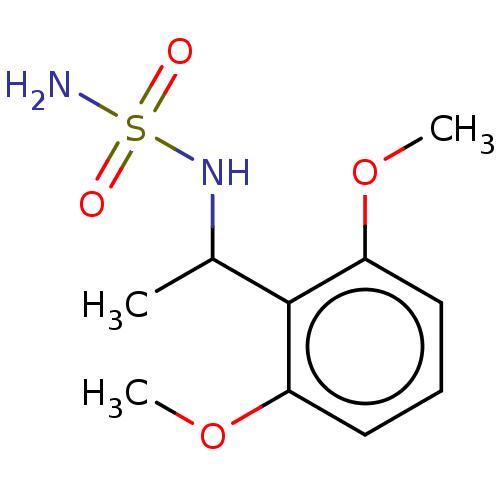
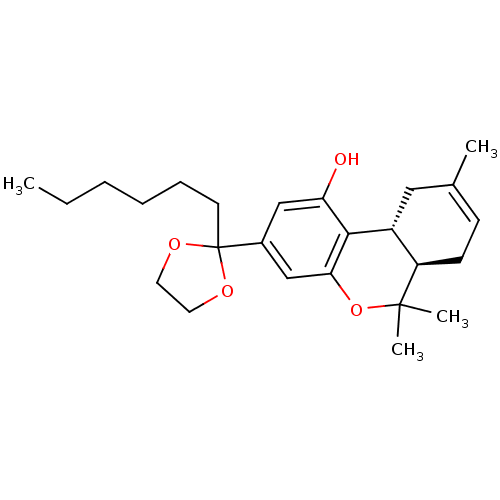
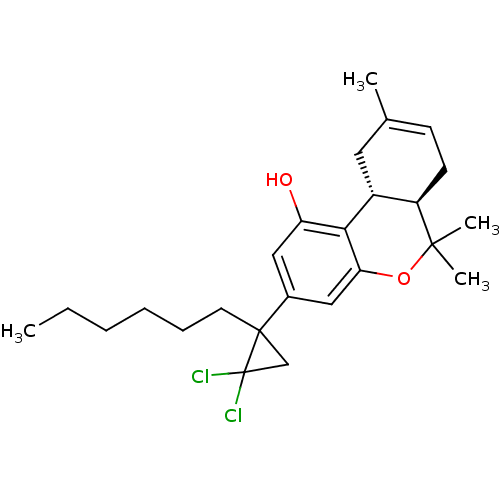
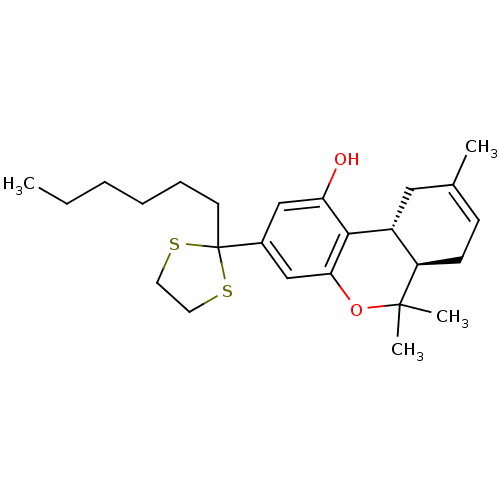
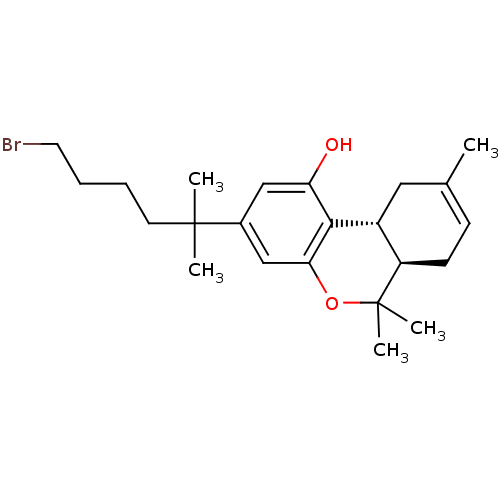
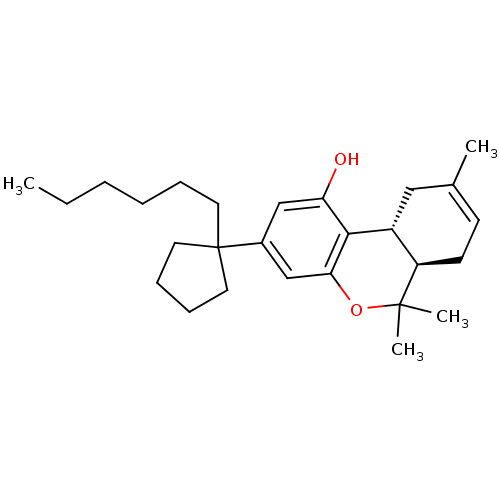
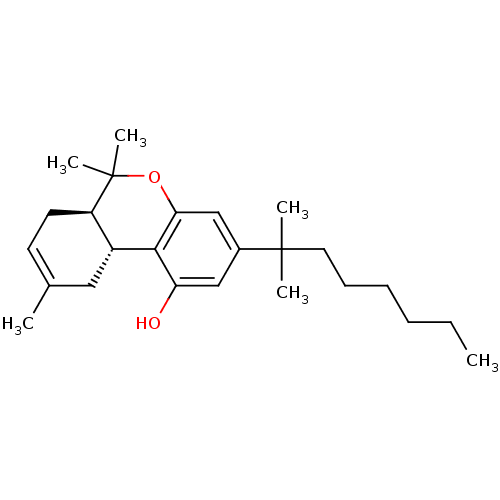
Affinity DataKi: 0.00500nMAssay Description:Inhibition of esterase activity of carbonic anhydrase 1 in human erythrocytes using PNF as substrate by spectrophotometer analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.00500nMAssay Description:Inhibition of esterase activity of carbonic anhydrase 2 in human erythrocytes using PNF as substrate by spectrophotometer analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.00500nMAssay Description:Inhibition of esterase activity of carbonic anhydrase 2 in human erythrocytes using PNF as substrate by spectrophotometer analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.00600nMAssay Description:Inhibition of esterase activity of carbonic anhydrase 2 in human erythrocytes using PNF as substrate by spectrophotometer analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.00600nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.00600nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.00800nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.0110nMAssay Description:Inhibition of esterase activity of carbonic anhydrase 1 in human erythrocytes using PNF as substrate by spectrophotometer analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.0230nMAssay Description:Inhibition of esterase activity of carbonic anhydrase 2 in human erythrocytes using PNF as substrate by spectrophotometer analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.0380nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.0460nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.0480nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.0490nMAssay Description:Inhibition of esterase activity of carbonic anhydrase 2 in human erythrocytes using PNF as substrate by spectrophotometer analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.0600nMAssay Description:Inhibition of esterase activity of carbonic anhydrase 2 in human erythrocytes using PNF as substrate by spectrophotometer analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.0890nMAssay Description:Inhibition of esterase activity of carbonic anhydrase 2 in human erythrocytes using PNF as substrate by spectrophotometer analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.105nMAssay Description:Inhibition of esterase activity of carbonic anhydrase 1 in human erythrocytes using PNF as substrate by spectrophotometer analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.127nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.127nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.137nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.140nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.156nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.193nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's methodMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Binding affinity to CB1 receptorMore data for this Ligand-Target Pair
TargetCannabinoid receptor 2(Homo sapiens (Human))
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataKi: 0.220nMAssay Description:Binding affinity to CB2 receptorMore data for this Ligand-Target Pair
TargetCannabinoid receptor 2(Homo sapiens (Human))
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataKi: 0.220nMAssay Description:Binding affinity to CB2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.275nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's methodMore data for this Ligand-Target Pair
TargetCannabinoid receptor 2(Homo sapiens (Human))
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataKi: 0.290nMAssay Description:Binding affinity to CB2 receptorMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataKi: 0.320nMAssay Description:Binding affinity to CB1 receptorMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataKi: 0.320nMAssay Description:Binding affinity to CB1 receptor (unknown origin)More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataKi: 0.324nMAssay Description:Binding affinity at CB1 receptorMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataKi: 0.340nMAssay Description:Binding affinity to CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.384nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's methodMore data for this Ligand-Target Pair
TargetCannabinoid receptor 2(Homo sapiens (Human))
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataKi: 0.390nMAssay Description:Binding affinity to CB2 receptorMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataKi: 0.430nMAssay Description:Binding affinity to CB1 receptorMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataKi: 0.437nMAssay Description:Binding affinity at CB1 receptorMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataKi: 0.440nMAssay Description:Binding affinity to CB1 receptorMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataKi: 0.447nMAssay Description:Binding affinity at CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.450nMAssay Description:Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur...More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataKi: 0.450nMAssay Description:Binding affinity to CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.454nMAssay Description:Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.479nMAssay Description:Inhibition of esterase activity of carbonic anhydrase 1 in human erythrocytes using PNF as substrate by spectrophotometer analysisMore data for this Ligand-Target Pair
TargetCannabinoid receptor 2(Homo sapiens (Human))
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataKi: 0.490nMAssay Description:Binding affinity to CB2 receptorMore data for this Ligand-Target Pair
TargetCannabinoid receptor 2(Homo sapiens (Human))
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataKi: 0.520nMAssay Description:Binding affinity to CB2 receptorMore data for this Ligand-Target Pair
TargetCannabinoid receptor 2(Homo sapiens (Human))
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataKi: 0.520nMAssay Description:Binding affinity to CB2 receptor (unknown origin)More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataKi: 0.520nMAssay Description:Binding affinity to CB1 receptorMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataKi: 0.525nMAssay Description:Binding affinity at CB1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.565nMAssay Description:Inhibition of esterase activity of carbonic anhydrase 1 in human erythrocytes using PNF as substrate by spectrophotometer analysisMore data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataKi: 0.570nMAssay Description:Binding affinity to CB1 receptorMore data for this Ligand-Target Pair
TargetCannabinoid receptor 2(Homo sapiens (Human))
The National Hellenic Research Foundation
Curated by ChEMBL
The National Hellenic Research Foundation
Curated by ChEMBL
Affinity DataKi: 0.650nMAssay Description:Binding affinity to CB2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.657nMAssay Description:Inhibition of esterase activity of carbonic anhydrase 1 in human erythrocytes using PNF as substrate by spectrophotometer analysisMore data for this Ligand-Target Pair