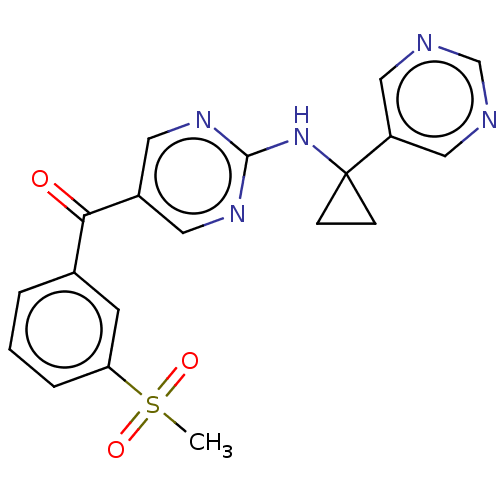
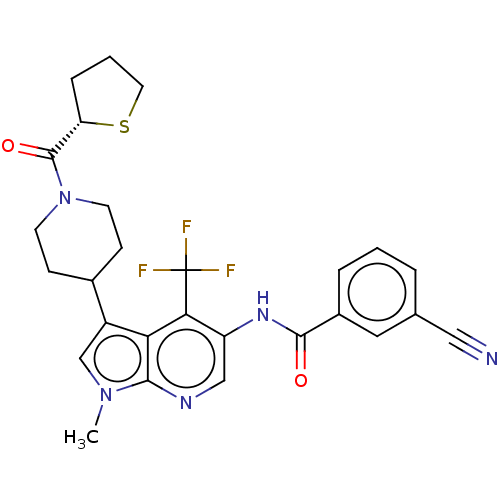
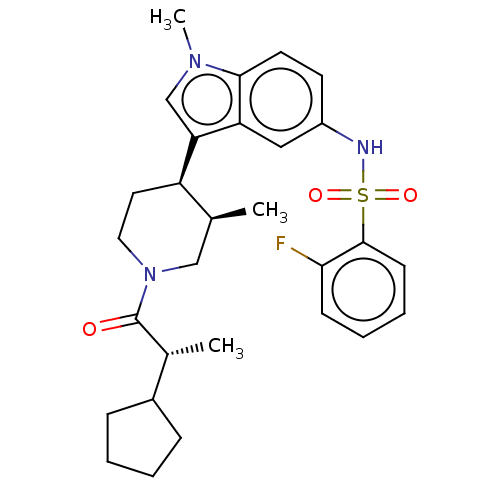
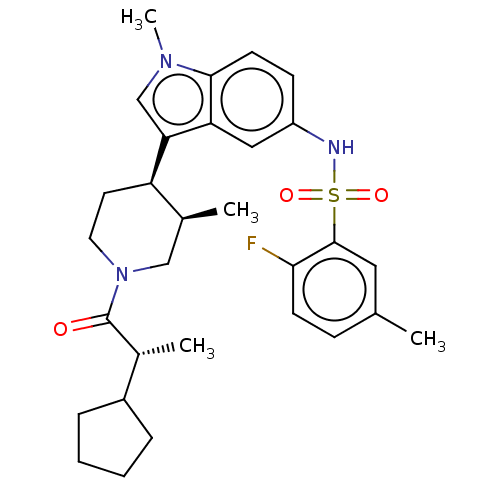
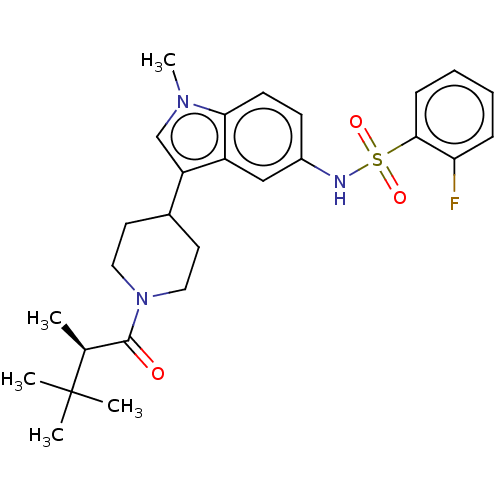
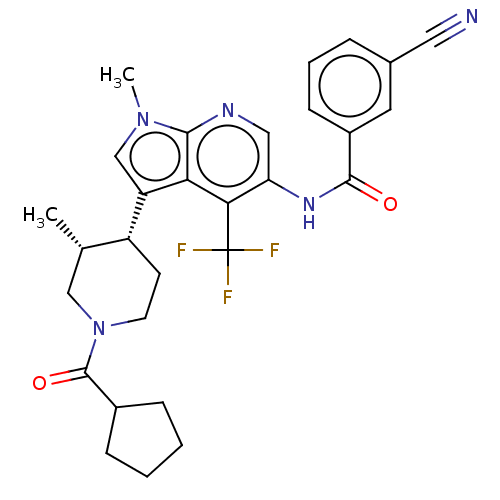
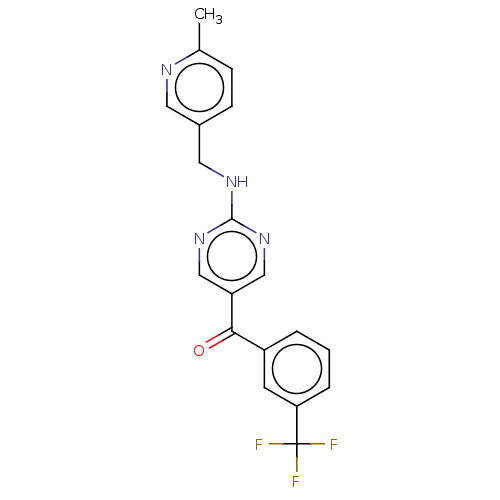
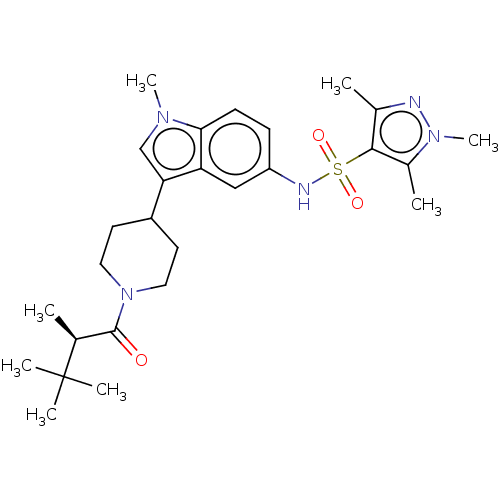
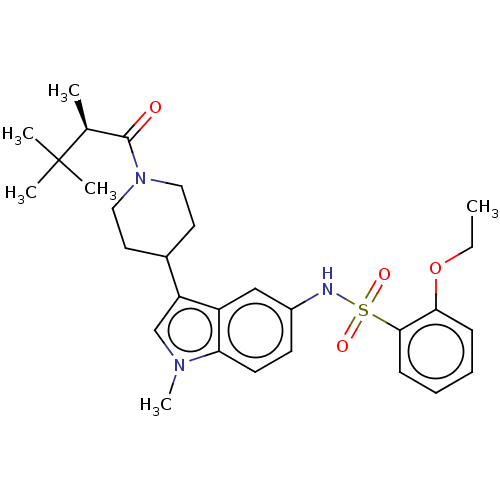
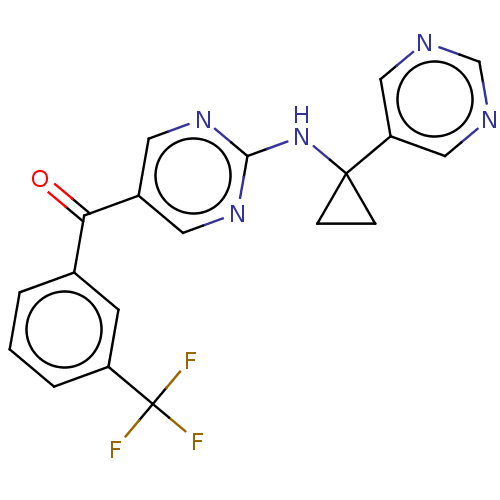
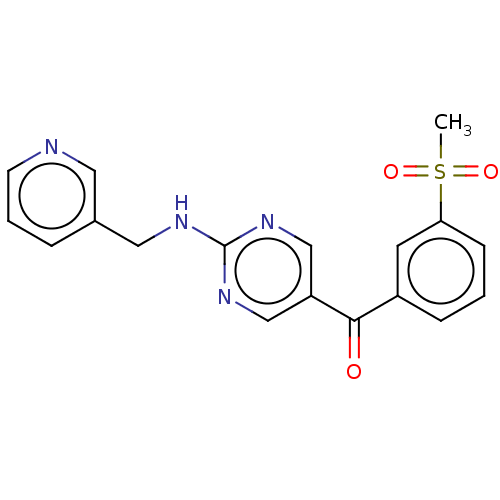
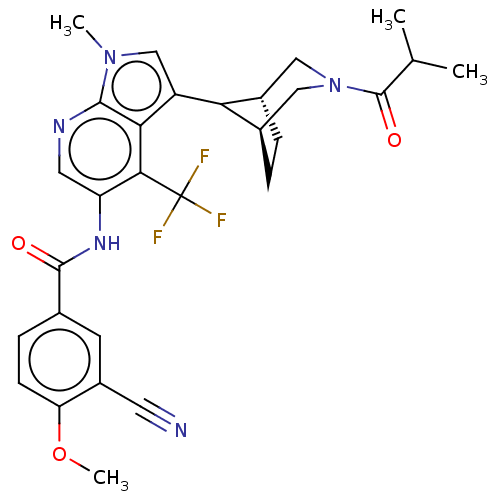
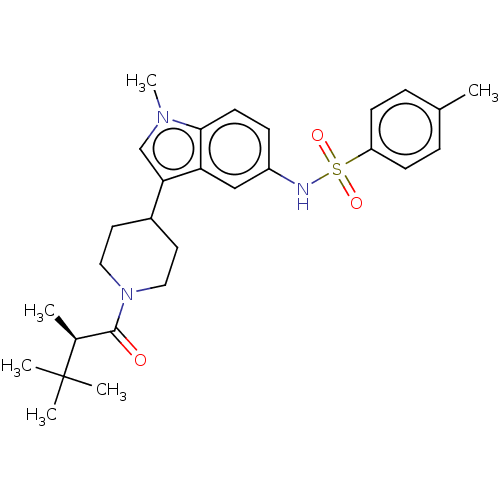
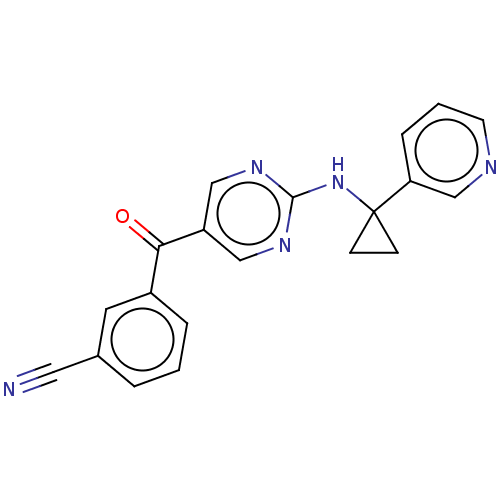
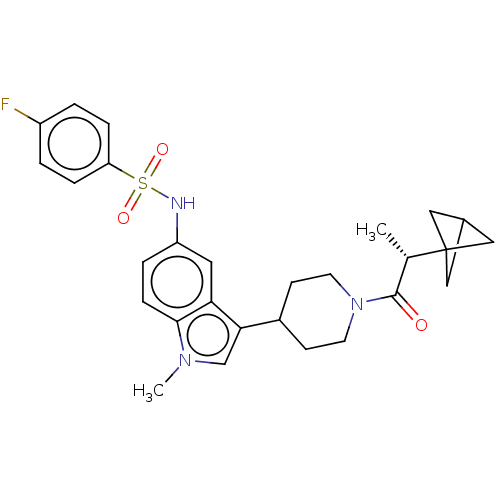
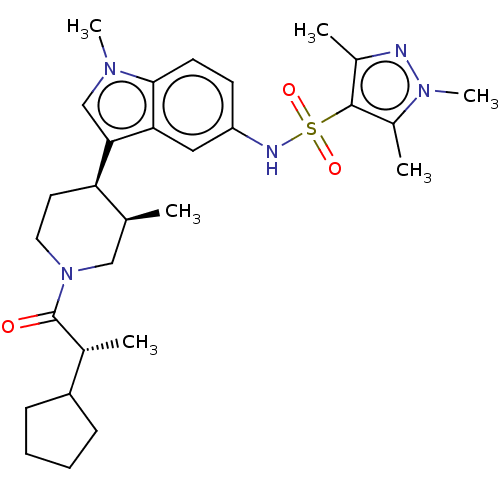
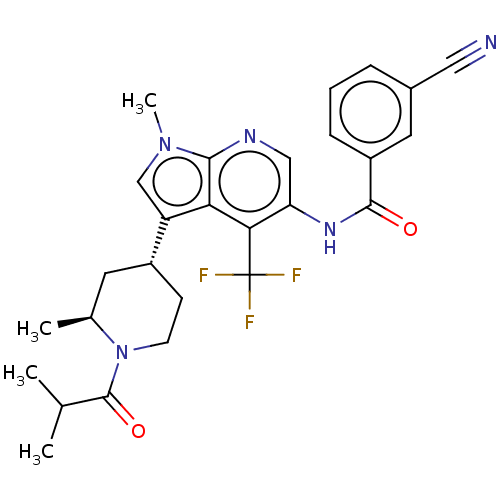
Affinity DataKi: 0.0850nMAssay Description:Displacement of [3H]Spiperone from dopamine D3 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 0.490nMAssay Description:Displacement of [3H]Spiperone from human dopamine D2L receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.690nMAssay Description:Displacement of [3H]Spiperone from human D2S receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Competitive inhibition of human SGLT2 expressed in CHO-K1 cells assessed as inhibition of [14C]-alpha-methylglucoside uptakeMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Antagonist potency against adenosine A2B receptor of guinea pig thoracic aortic smooth muscleMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell...More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell...More data for this Ligand-Target Pair
Affinity DataKi: 50nMAssay Description:Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell...More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nMAssay Description:Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell...More data for this Ligand-Target Pair
Affinity DataKi: 1.80E+3nMAssay Description:Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist]More data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist]More data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist]More data for this Ligand-Target Pair
Affinity DataKi: 4.52E+3nMAssay Description:Antagonist potency at cloned recombinant human adenosine A2B receptor transfected in CHO cells by cAMP assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.10E+4nMAssay Description:Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist]More data for this Ligand-Target Pair
Affinity DataKi: 1.70E+4nMAssay Description:Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist]More data for this Ligand-Target Pair
Affinity DataKi: 1.90E+4nMAssay Description:Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell...More data for this Ligand-Target Pair
Affinity DataKi: 1.90E+4nMAssay Description:Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist]More data for this Ligand-Target Pair
Affinity DataKi: 3.70E+4nMAssay Description:Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist]More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of VEGF2 receptor (unknown origin) by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Inhibition of human recombinant DPP4 using Gly-Pro-7-amido-4-methyl-coumarin as substrate incubated for 15 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:The vanin-1 protein was prepared in-house from a construct expressing the extracellular domain of human vanin-1 (GenBank ID NM_004666) preceded N-ter...More data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair






 3D Structure (crystal)
3D Structure (crystal)