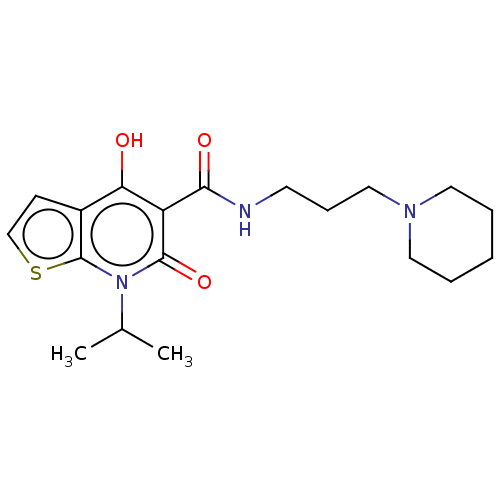
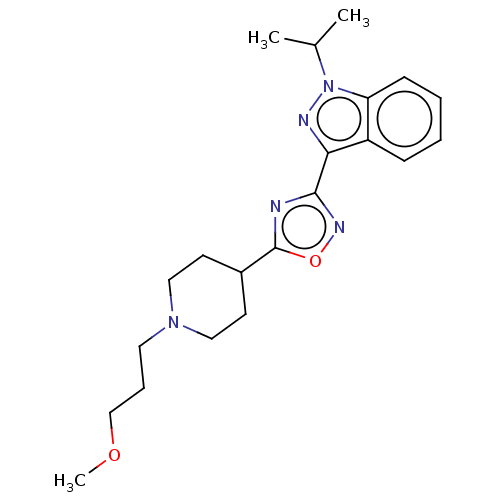
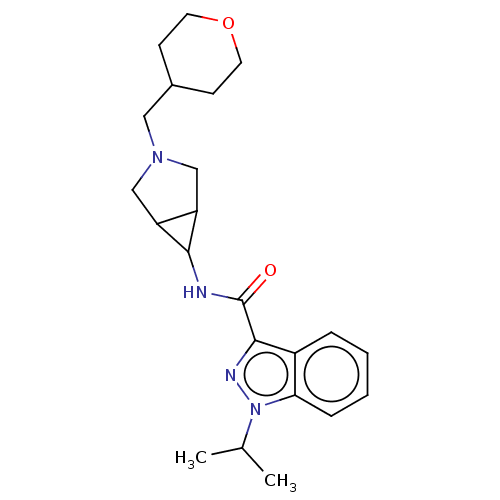
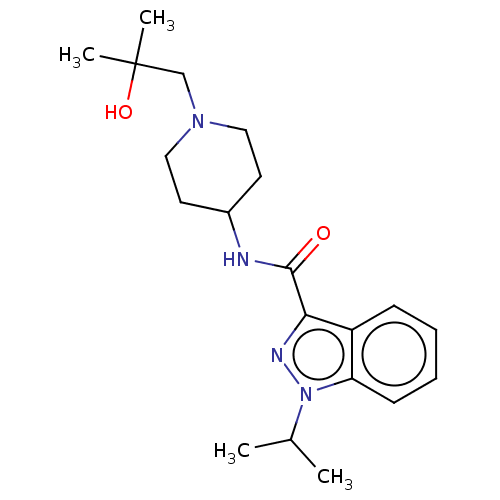
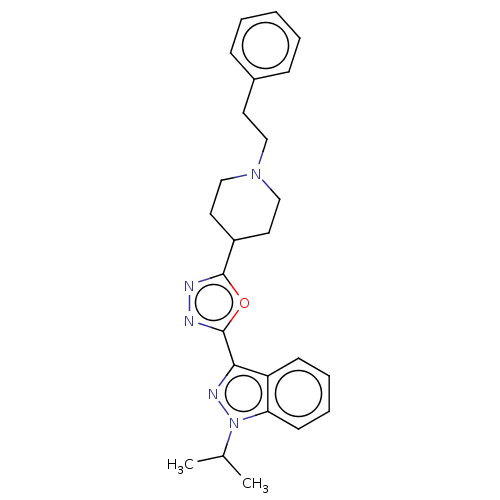
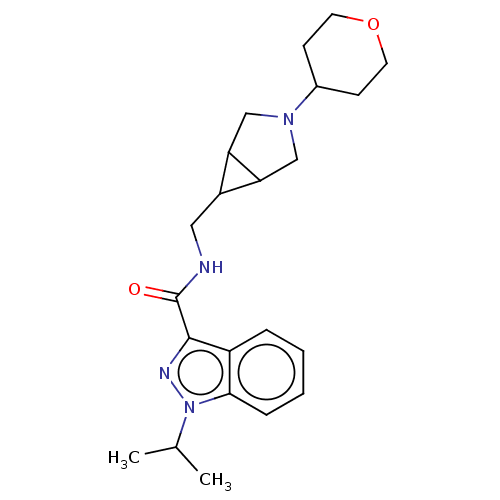
Affinity DataIC50: 950nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibitory activity against Dihydrofolate reductase in rat liverMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of human ERG expressed in HEK293 cells by patch clamp methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of human ERG expressed in HEK293 cells by patch clamp methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig left atriaMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 8.50E+3nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 9.10E+3nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 9.10E+3nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of rat microsomal HMG-CoA reductase activity by 50%More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 12 mins in presence of NADPH-regeneration system by LC-MS/MS...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 2 mins in presence of NADPH-regeneration system by LC-MS/MS methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 12 mins in presence of NADPH-regeneration system by LC-MS/MS...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 2 mins in presence of NADPH-regeneration system by LC-MS/MS methodMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Evaluated for inhibitory activity against rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.12E+4nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.39E+4nMAssay Description:The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig right atriaMore data for this Ligand-Target Pair
Affinity DataIC50: 2.04E+4nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.67E+4nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+4nMAssay Description:The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig left atriaMore data for this Ligand-Target Pair
Affinity DataIC50: 3.64E+4nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70E+4nMAssay Description:The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig right atriaMore data for this Ligand-Target Pair
Affinity DataIC50: 3.77E+4nMAssay Description:The compound was tested for beta-adrenergic activity against Beta-2 adrenergic receptor from guinea pig trachea.More data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:The compound was tested for alpha-adrenergic activity against Alpha-1 adrenergic receptor from rat aorta.More data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig left atriaMore data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig right atriaMore data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig left atriaMore data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig left atriaMore data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig right atriaMore data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig right atriaMore data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:Antagonistic activity against Thromboxane A2 receptor in guinea pig trachea in the presence of 11,9-epoxymethano-PGH2More data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:Evaluated for inhibitory activity against rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >4.50E+4nMAssay Description:Inhibition of rabbit lung angiotensin I converting enzyme (ACE) using hippuryl-histidyl-leucine as substrateMore data for this Ligand-Target Pair