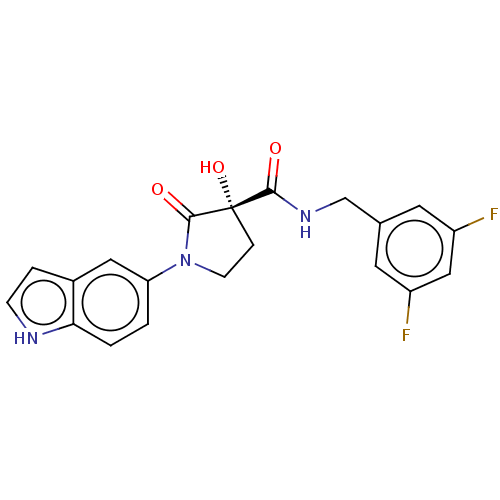
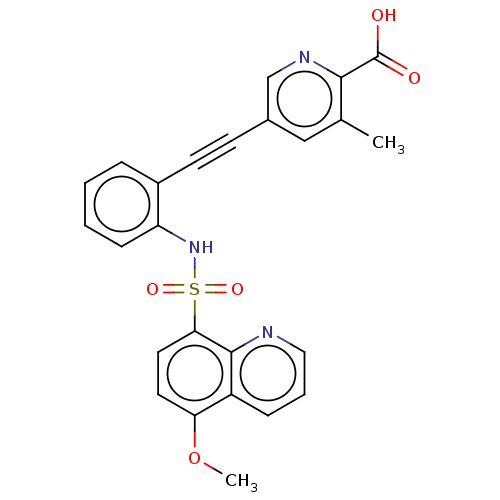
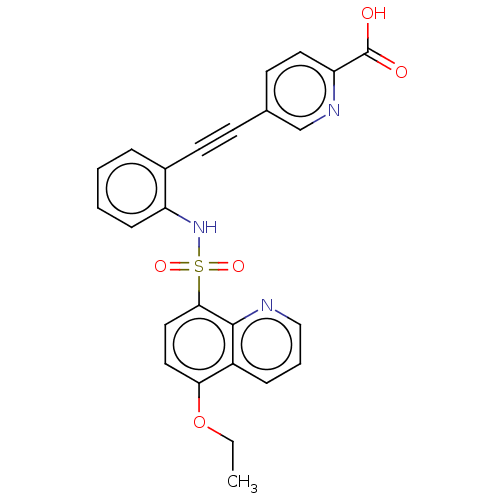
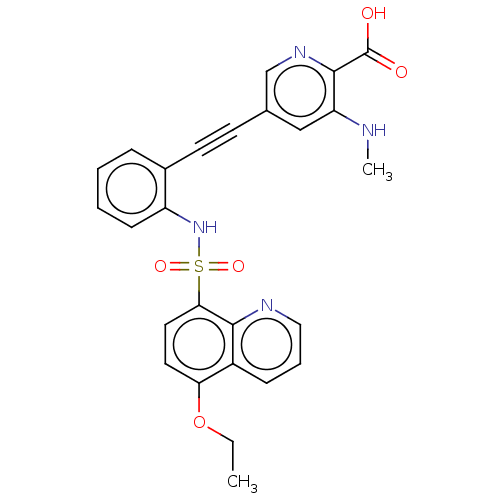
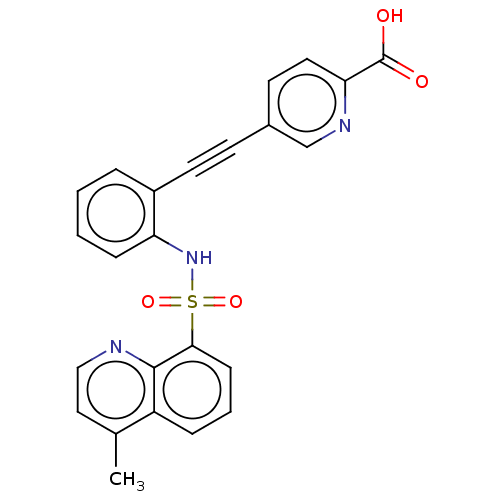
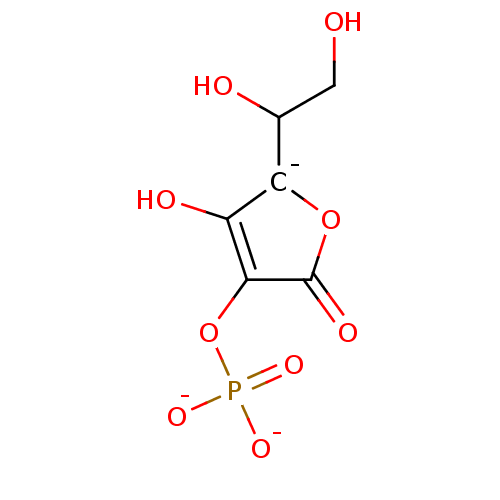
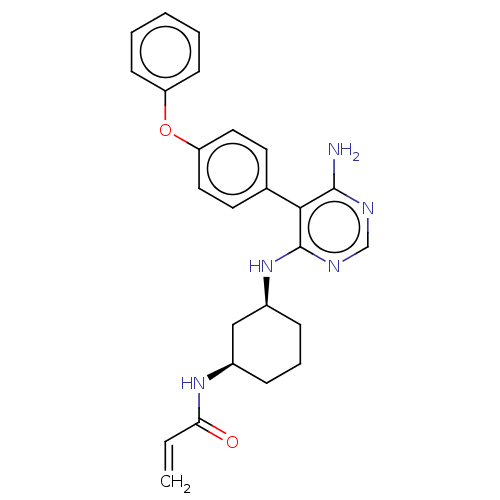
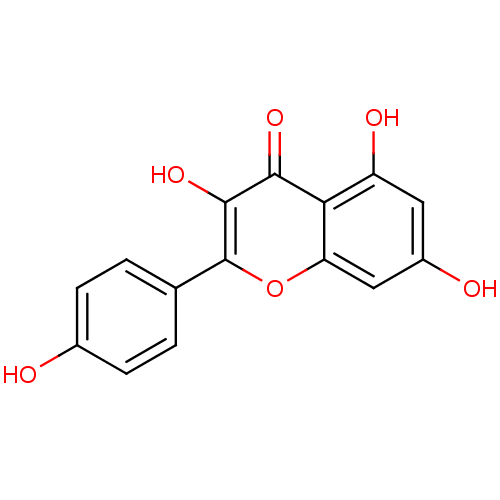
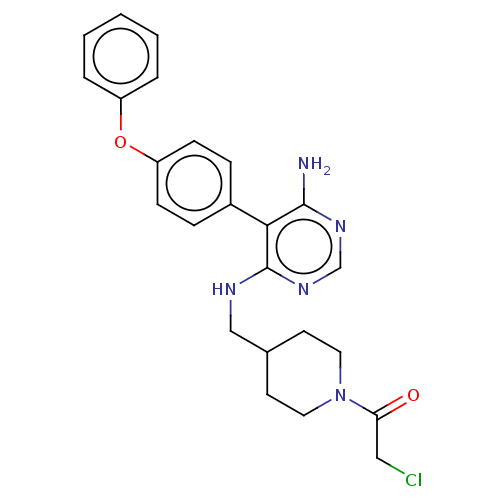
Affinity DataKi: 0.160nMAssay Description:Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b...More data for this Ligand-Target Pair
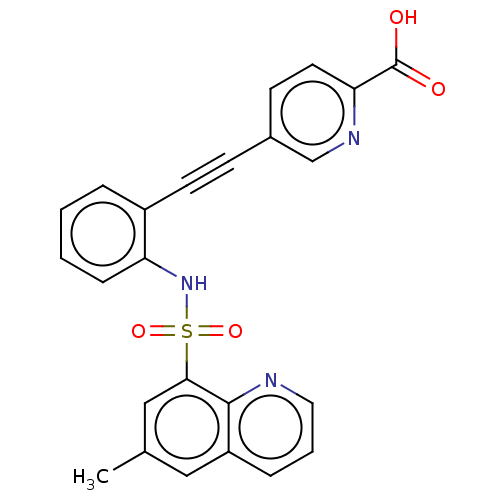
Affinity DataKi: 0.160nMAssay Description:Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b...More data for this Ligand-Target Pair
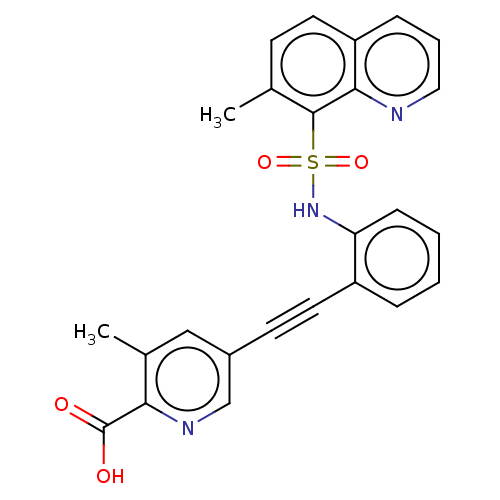
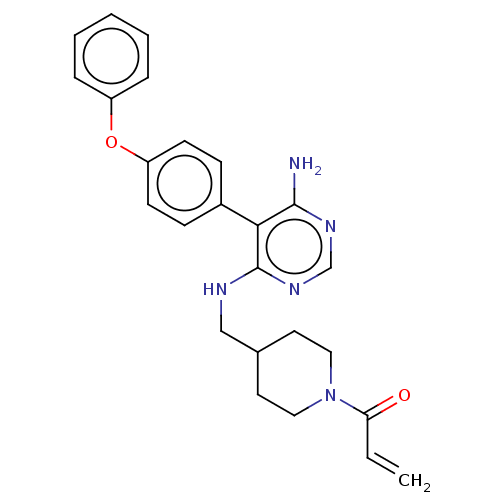
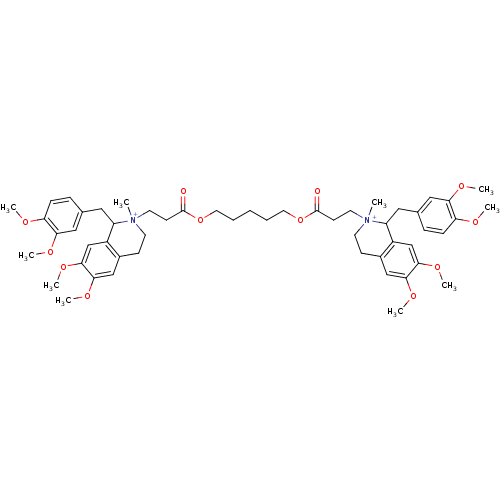
Affinity DataKi: 0.900nMAssay Description:Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b...More data for this Ligand-Target Pair
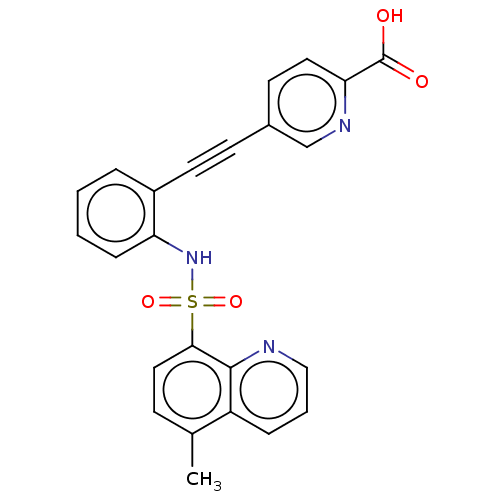
Affinity DataKi: 0.900nMAssay Description:Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b...More data for this Ligand-Target Pair
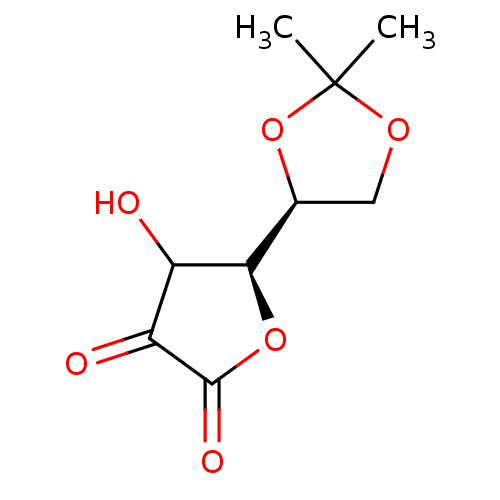
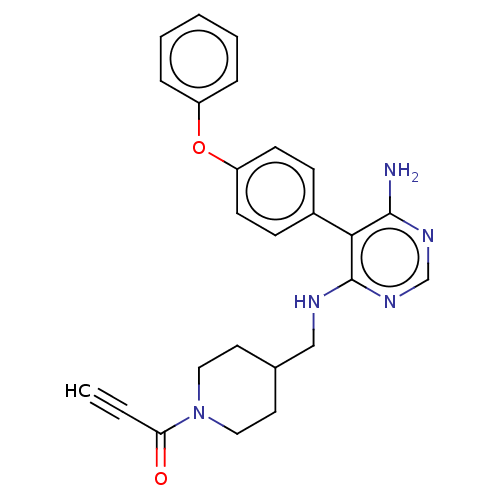
Affinity DataKi: 3.40nMAssay Description:Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b...More data for this Ligand-Target Pair
Affinity DataKi: 3.40nMAssay Description:Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b...More data for this Ligand-Target Pair
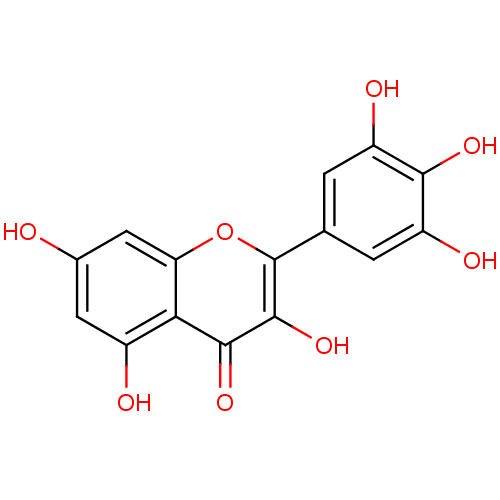
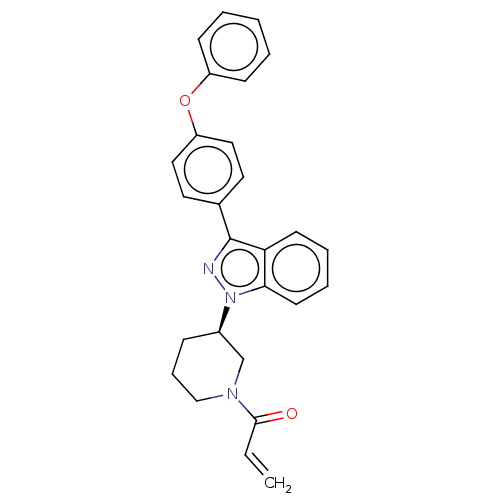
Affinity DataKi: 4.30nMAssay Description:Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b...More data for this Ligand-Target Pair
Affinity DataKi: 4.30nMAssay Description:Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b...More data for this Ligand-Target Pair
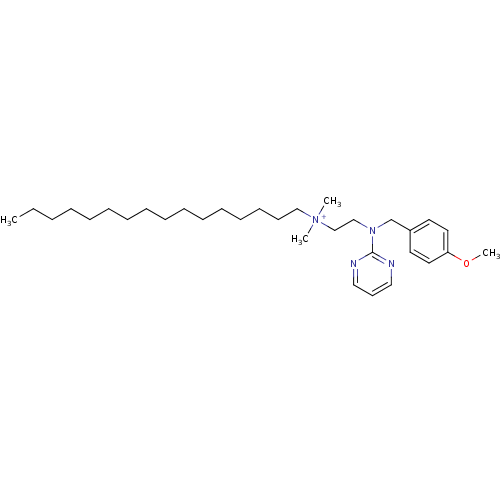
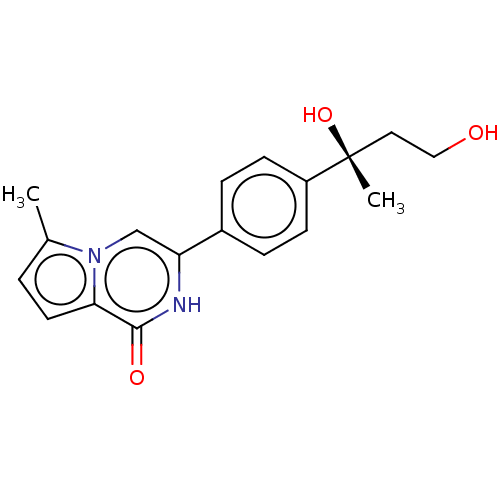
Affinity DataKi: 11nMAssay Description:Binding affinity to full-length C-terminal GFP-tagged human MCT4 incubated for 1 hrs by fluorescence cross-correlation spectroscopy analysisMore data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 11nMAssay Description:Binding affinity to full-length C-terminal GFP-tagged human MCT4 incubated for 1 hrs by fluorescence cross-correlation spectroscopy analysisMore data for this Ligand-Target Pair
Ligand Info
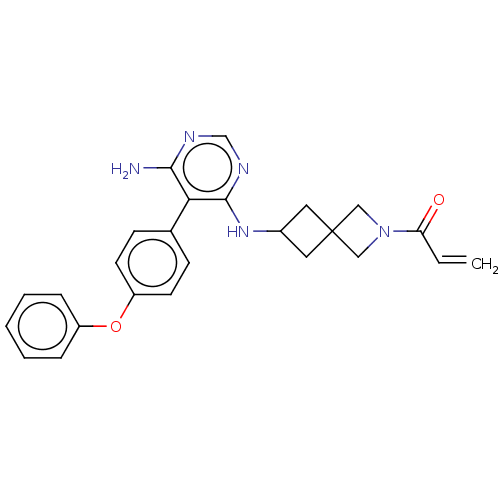
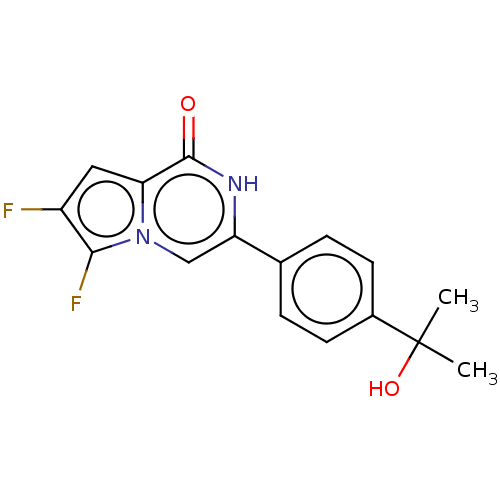
Affinity DataKi: 16nMAssay Description:Binding affinity to full-length C-terminal GFP-tagged human MCT4 incubated for 1 hrs by fluorescence cross-correlation spectroscopy analysisMore data for this Ligand-Target Pair
Ligand Info
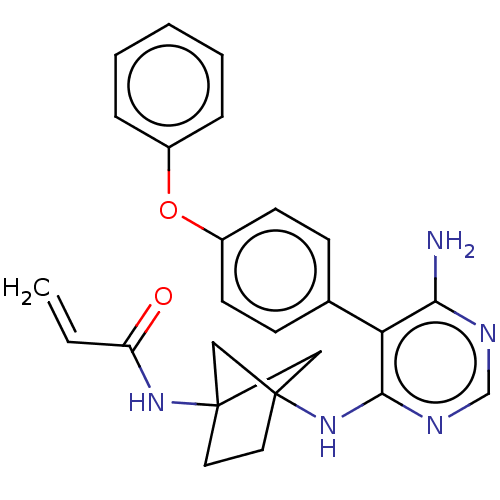
Affinity DataKi: 17nMAssay Description:Binding affinity to full-length C-terminal GFP-tagged human MCT4 incubated for 1 hrs by fluorescence cross-correlation spectroscopy analysisMore data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 17nMAssay Description:Binding affinity to full-length C-terminal GFP-tagged human MCT4 incubated for 1 hrs by fluorescence cross-correlation spectroscopy analysisMore data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 17nMAssay Description:Binding affinity to full-length C-terminal GFP-tagged human MCT4 incubated for 1 hrs by fluorescence cross-correlation spectroscopy analysisMore data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 29nMAssay Description:Binding affinity to full-length C-terminal GFP-tagged human MCT4 incubated for 1 hrs by fluorescence cross-correlation spectroscopy analysisMore data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 32nMAssay Description:Binding affinity to full-length C-terminal GFP-tagged human MCT4 incubated for 1 hrs by fluorescence cross-correlation spectroscopy analysisMore data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 37nMAssay Description:Binding affinity to full-length C-terminal GFP-tagged human MCT4 incubated for 1 hrs by fluorescence cross-correlation spectroscopy analysisMore data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 51nMAssay Description:Binding affinity to full-length C-terminal GFP-tagged human MCT4 incubated for 1 hrs by fluorescence cross-correlation spectroscopy analysisMore data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 56nMAssay Description:Binding affinity to full-length C-terminal GFP-tagged human MCT4 incubated for 1 hrs by fluorescence cross-correlation spectroscopy analysisMore data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 99nMAssay Description:Binding affinity to full-length C-terminal GFP-tagged human MCT4 incubated for 1 hrs by fluorescence cross-correlation spectroscopy analysisMore data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 222nMAssay Description:Binding affinity to full-length C-terminal GFP-tagged human MCT4 incubated for 1 hrs by fluorescence cross-correlation spectroscopy analysisMore data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 301nMAssay Description:Binding affinity to full-length C-terminal GFP-tagged mouse MCT4 incubated for 1 hrs by fluorescence cross-correlation spectroscopy analysisMore data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 320nMAssay Description:The inhibitor screening assays were performed in 96-well format with 1152 small molecules found in the Prestwick chemical library (Prestwick Chemical...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 530nMAssay Description:Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataKi: 590nMAssay Description:The inhibitor screening assays were performed in 96-well format with 1152 small molecules found in the Prestwick chemical library (Prestwick Chemical...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 780nMAssay Description:Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 1.30E+3nMAssay Description:Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 1.80E+3nMAssay Description:Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataKi: 3.10E+3nMAssay Description:Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3.27E+3nMAssay Description:The inhibitor screening assays were performed in 96-well format with 1152 small molecules found in the Prestwick chemical library (Prestwick Chemical...More data for this Ligand-Target Pair
Affinity DataKi: 1.48E+4nMAssay Description:The inhibitor screening assays were performed in 96-well format with 1152 small molecules found in the Prestwick chemical library (Prestwick Chemical...More data for this Ligand-Target Pair
Affinity DataKi: 1.54E+5nMAssay Description:The inhibitor screening assays were performed in 96-well format with 1152 small molecules found in the Prestwick chemical library (Prestwick Chemical...More data for this Ligand-Target Pair
Affinity DataKi: 1.90E+5nMAssay Description:The inhibitor screening assays were performed in 96-well format with 1152 small molecules found in the Prestwick chemical library (Prestwick Chemical...More data for this Ligand-Target Pair
Affinity DataKi: 3.42E+5nMAssay Description:The inhibitor screening assays were performed in 96-well format with 1152 small molecules found in the Prestwick chemical library (Prestwick Chemical...More data for this Ligand-Target Pair
Affinity DataKi: 3.80E+5nMAssay Description:The inhibitor screening assays were performed in 96-well format with 1152 small molecules found in the Prestwick chemical library (Prestwick Chemical...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inhibitory activity against dihydrofolate reductase (DHFR) from rat liverMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoeaMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Inhibitory activity against dihydrofolate reductase (DHFR) of Plasmodium bergheiMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Covalent inhibition of N-terminal GST-fused human BTK (2-659(end) amino acids) expressed in baculovirus expression system using FITC-AHA-EEPLYWSFPAKK...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoeaMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Inhibitory activity against dihydrofolate reductase (DHFR) from Escherichia coliMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of N-terminal GST-tagged human EGFR (696 to end aminoacids) expressed in baculovirus infected Sf21 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibitory activity against dihydrofolate reductase (DHFR) from rat liverMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibitory activity against dihydrofolate reductase (DHFR) from rat liverMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoeaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Inhibitory activity against dihydrofolate reductase (DHFR) of Plasmodium bergheiMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Inhibitory activity against dihydrofolate reductase (DHFR) from Escherichia coliMore data for this Ligand-Target Pair

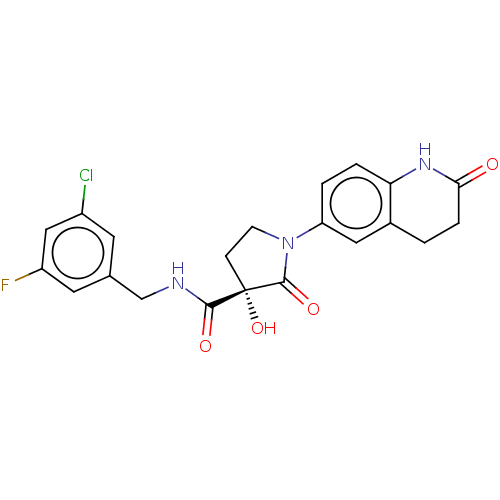
 3D Structure (crystal)
3D Structure (crystal)