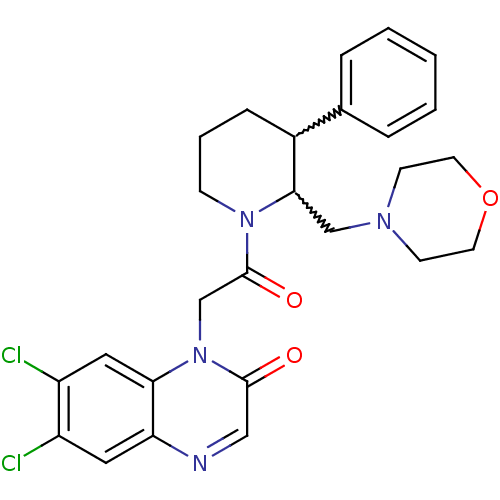
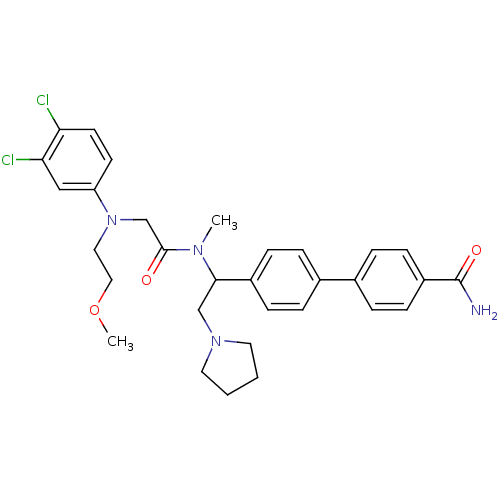
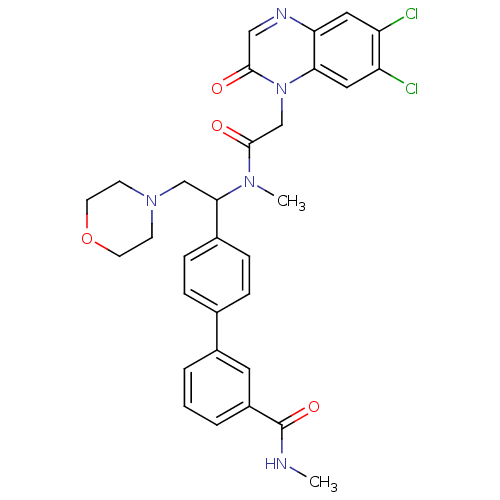
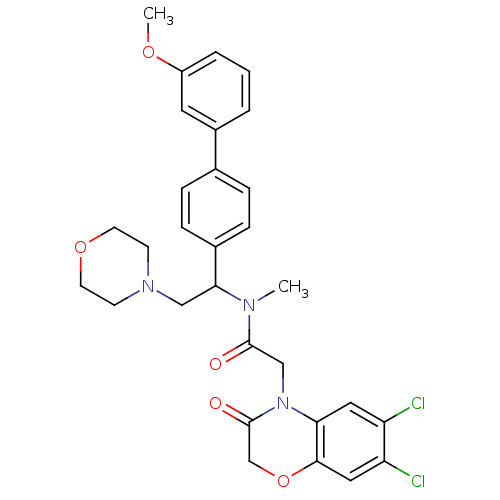
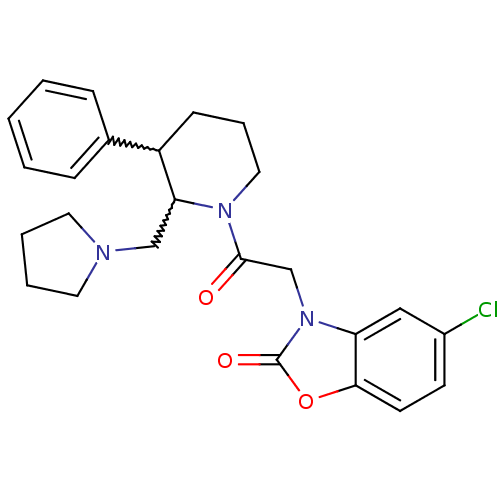
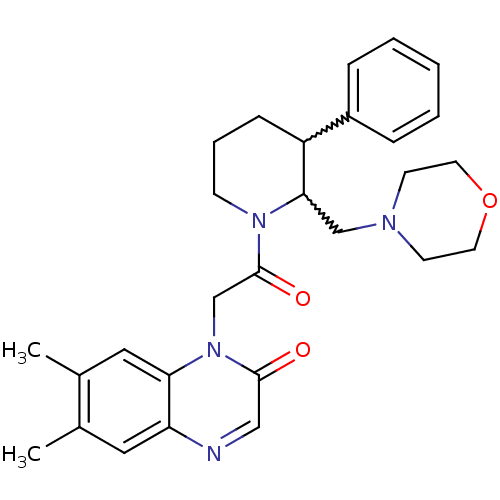
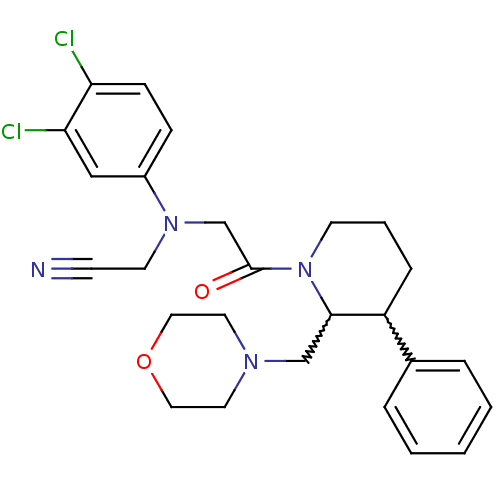
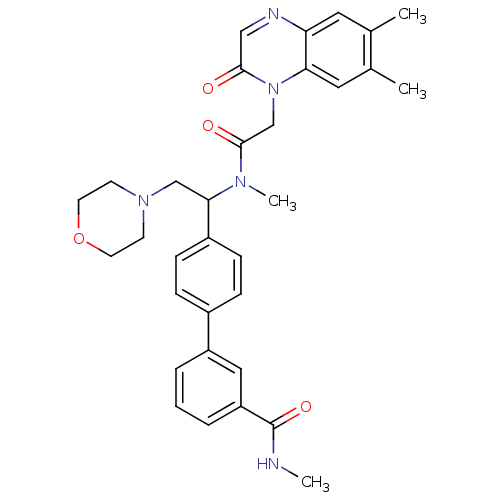
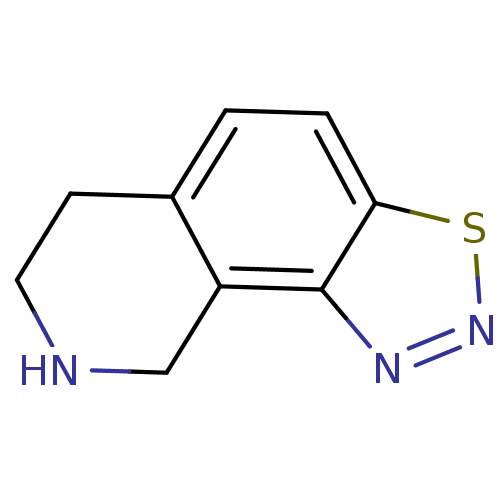
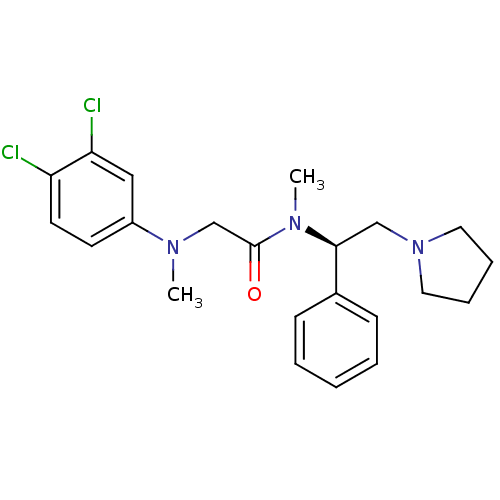
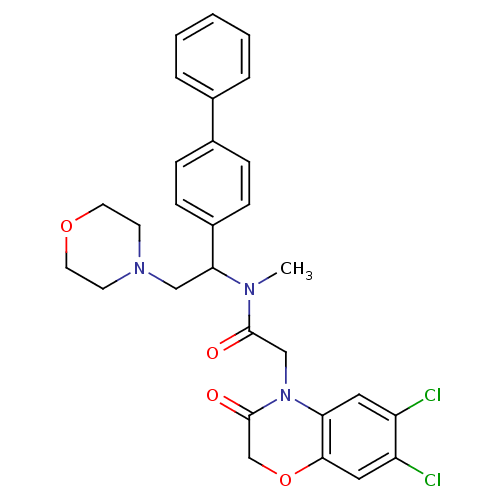
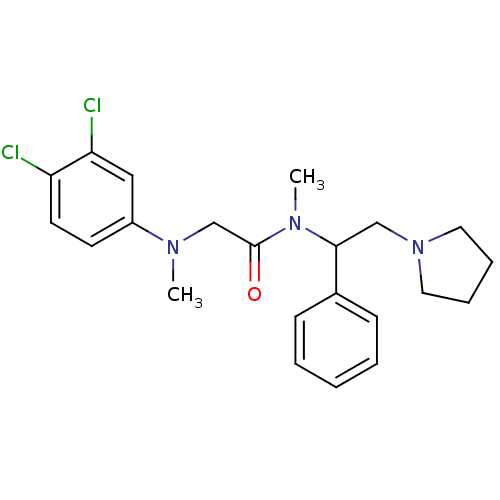
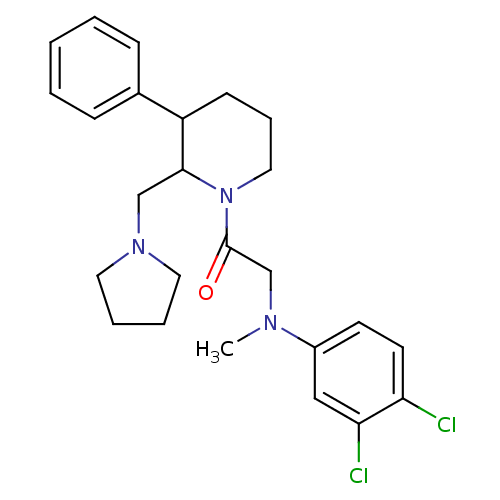
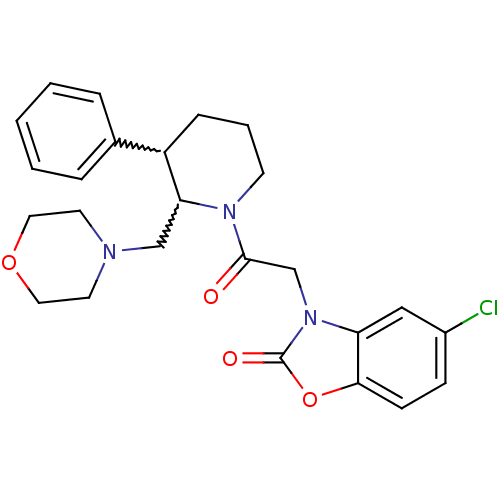
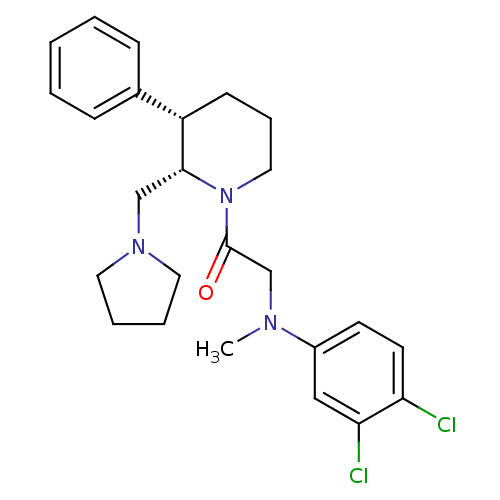
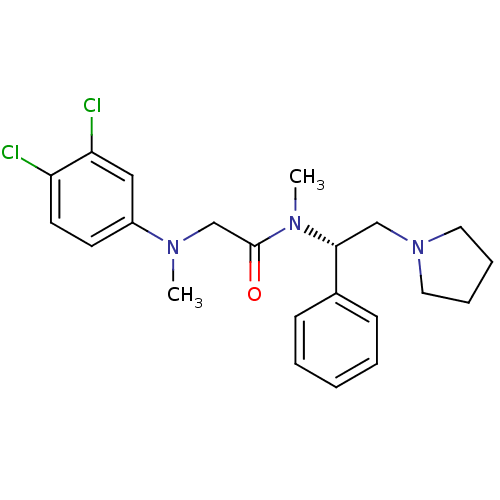
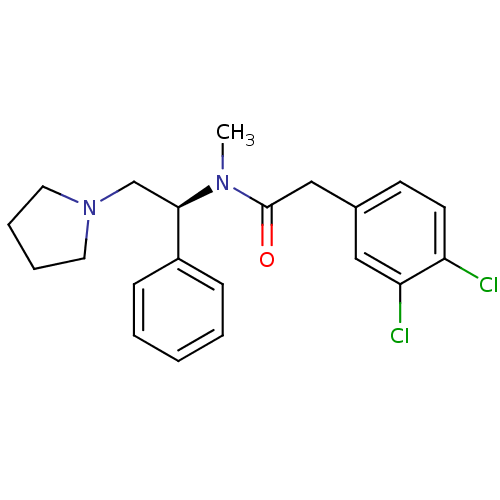
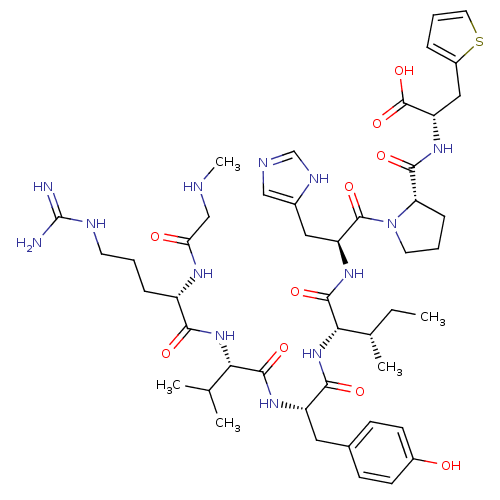
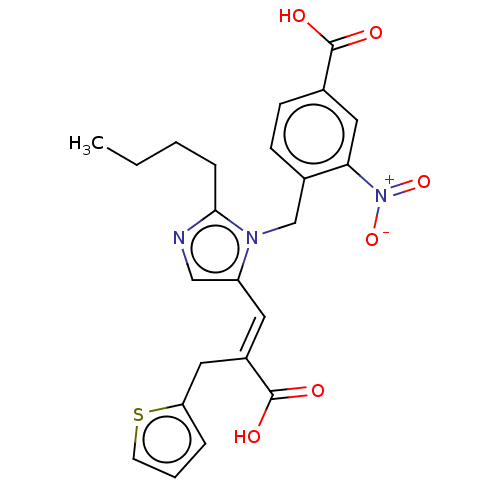
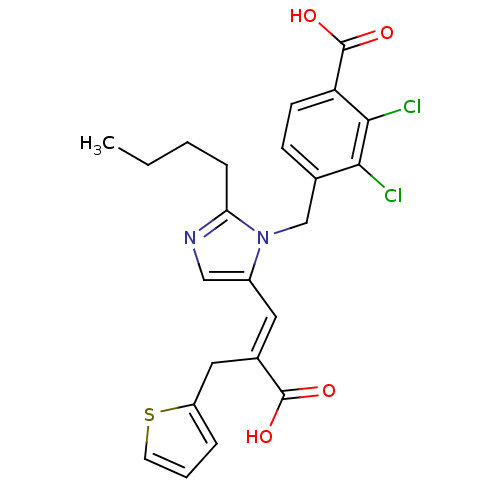
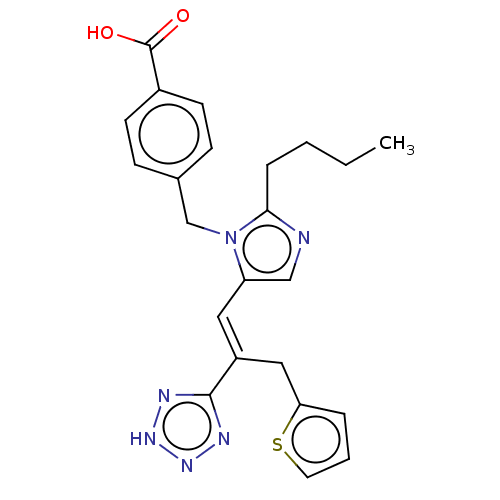
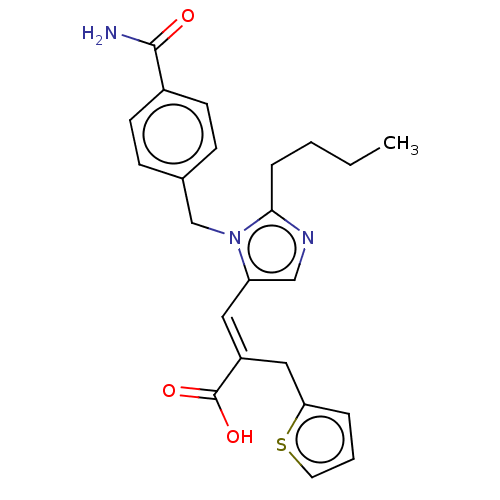
Affinity DataKi: 0.300nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Dissociation constant(Ki) of compound was determined to measure PNMT-inhibitory potencyMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Binding affinity for phenylethanolamine N-methyl-transferase was determined.More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Bos taurus (bovine))
Smith Kline & French Laboratories
Curated by ChEMBL
Smith Kline & French Laboratories
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Binding affinity for phenylethanolamine N-methyl-transferase was determined.More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferaseMore data for this Ligand-Target Pair
Affinity DataKi: 29nMAssay Description:Dissociation constant(Ki) of compound was determined to measure PNMT-inhibitory potencyMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferaseMore data for this Ligand-Target Pair
Affinity DataKi: 40nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 52nMAssay Description:Dissociation constant(Ki) of compound was determined to measure Phenylethanolamine N-methyltransferase inhibitory potencyMore data for this Ligand-Target Pair
Affinity DataKi: 59nMAssay Description:Dissociation constant(Ki) of compound was determined to measure PNMT-inhibitory potencyMore data for this Ligand-Target Pair
Affinity DataKi: 79nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 630nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 630nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.50E+3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Binding affinity to human urotensin2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.110nMAssay Description:In vitro inhibition of [125I]-AII specific binding towards angiotensin II receptor in rat mesenteric membranes.More data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor(RAT)
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.150nMAssay Description:Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteriesMore data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50More data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor(RAT)
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteriesMore data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor(RAT)
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteriesMore data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor(RAT)
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.560nMAssay Description:Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteriesMore data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor(RAT)
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Smithkline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.590nMAssay Description:Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteriesMore data for this Ligand-Target Pair

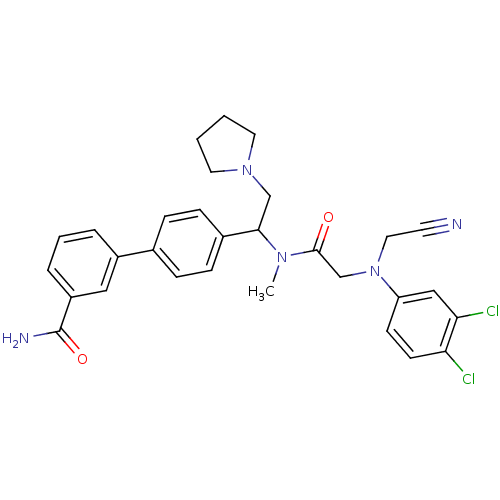
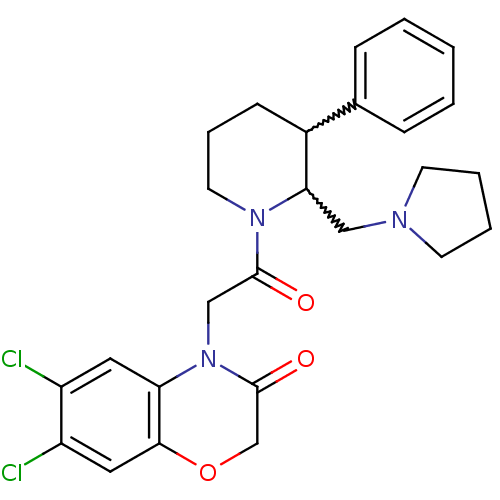
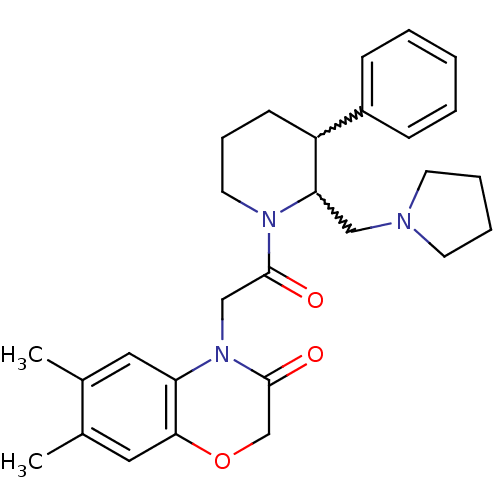
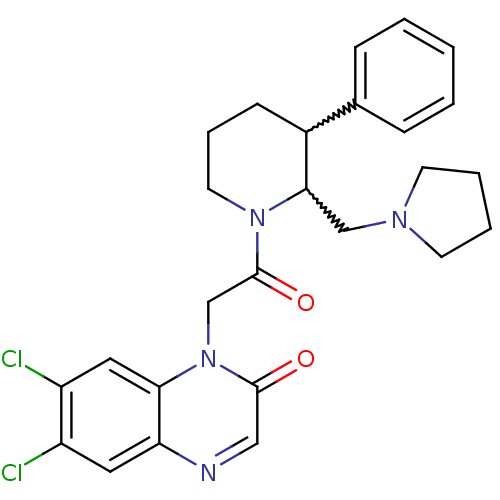
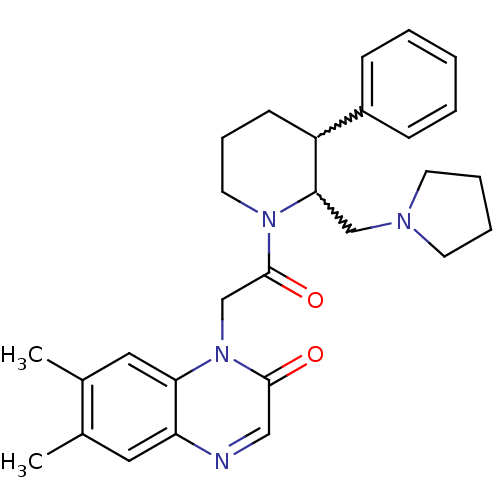
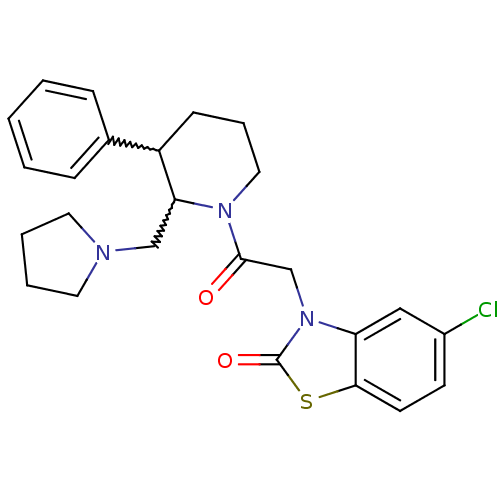
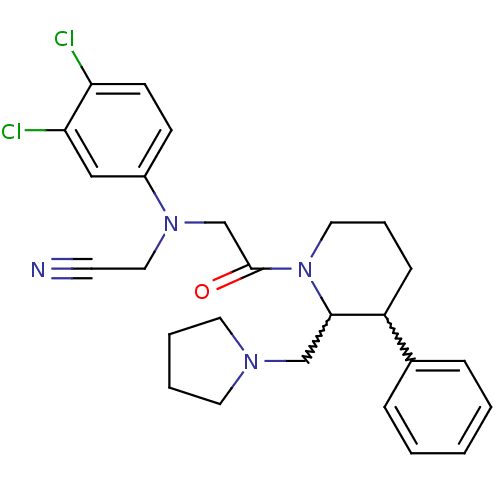
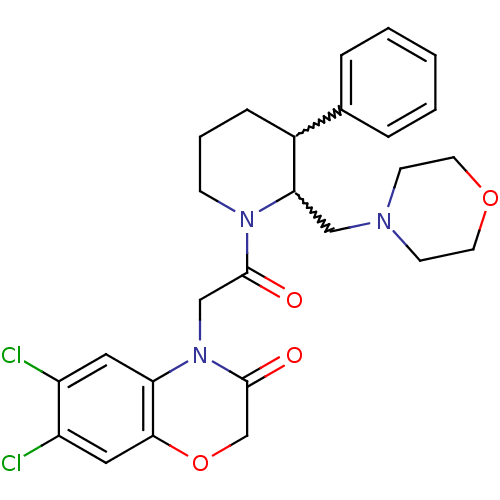
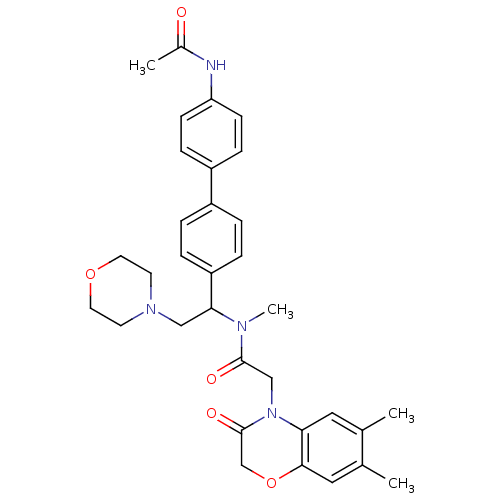
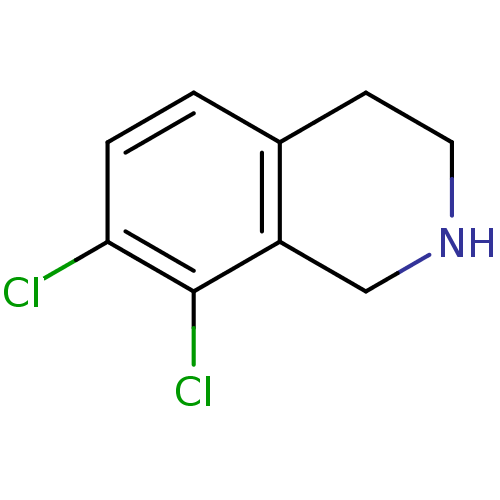
 3D Structure (crystal)
3D Structure (crystal)