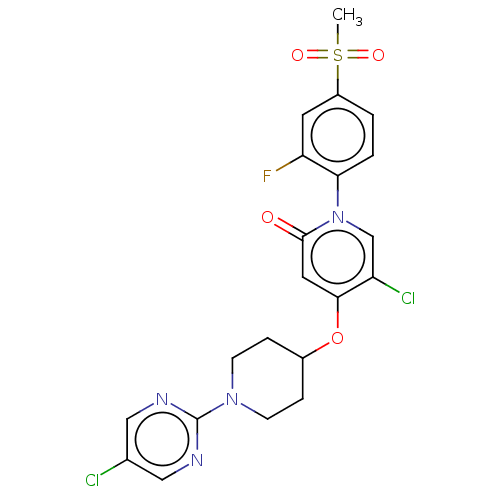
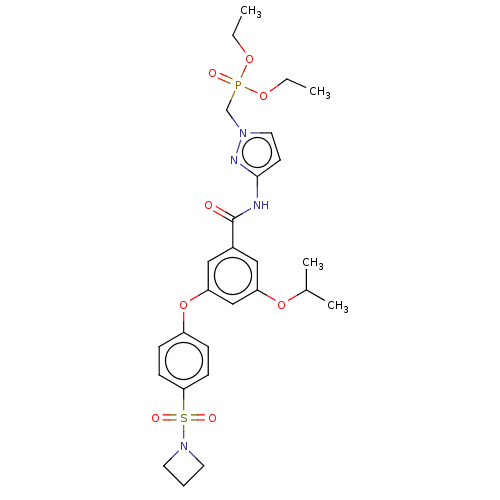
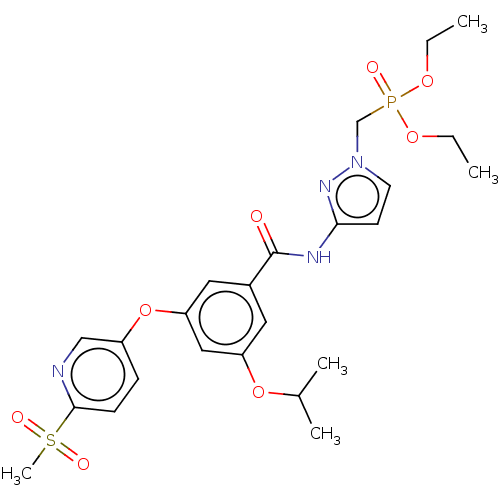
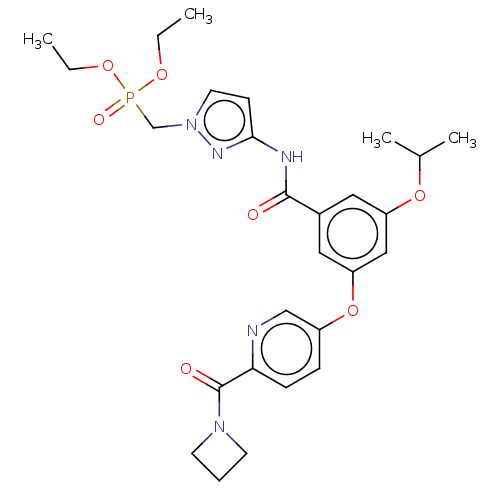
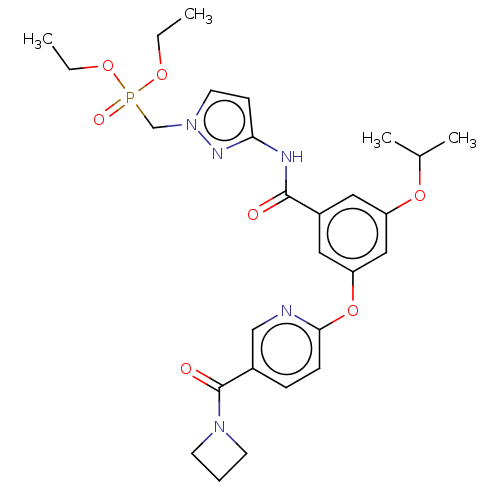
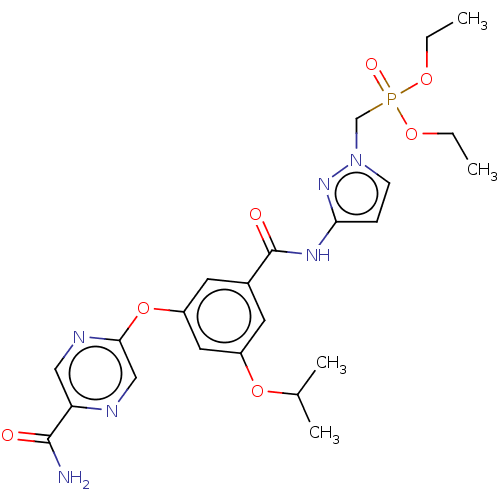
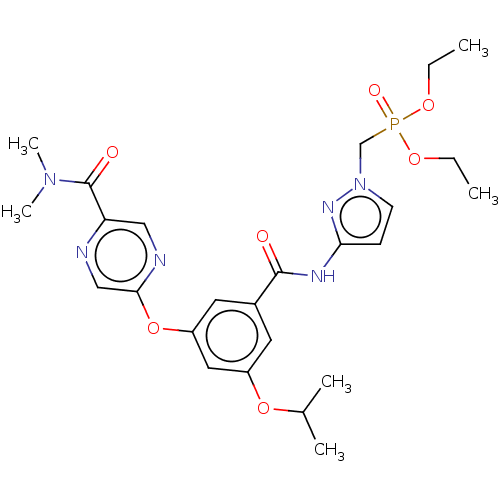
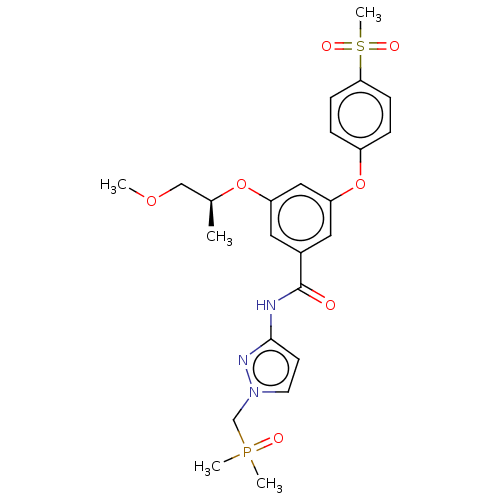
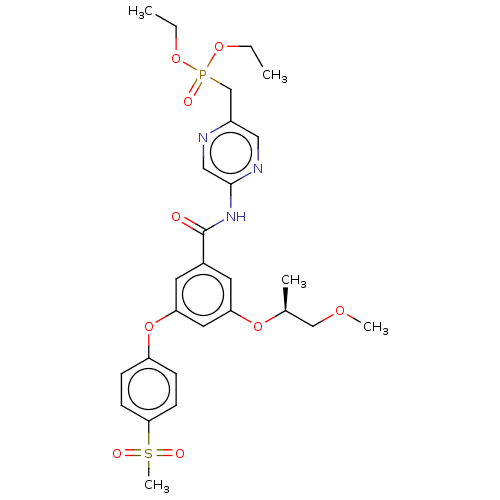
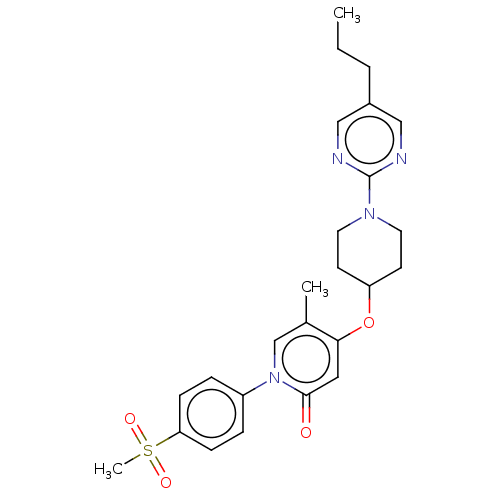
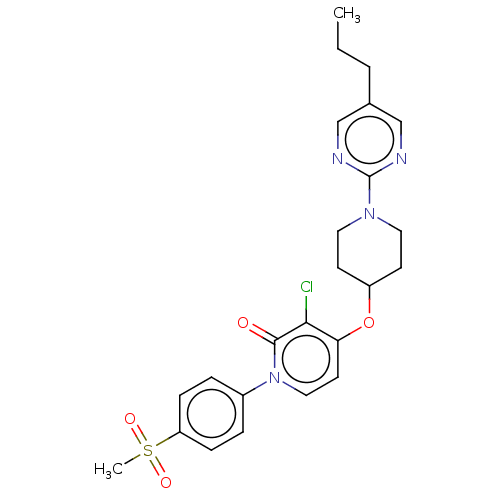
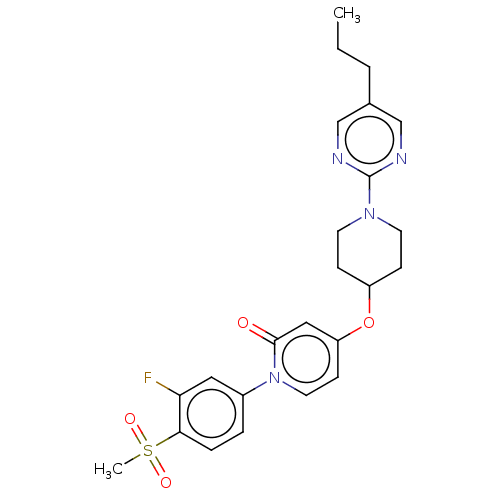
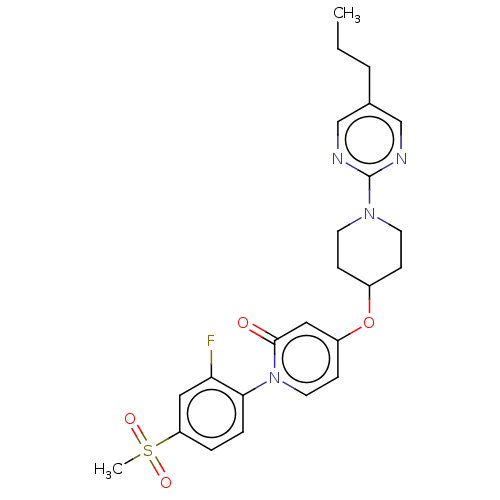
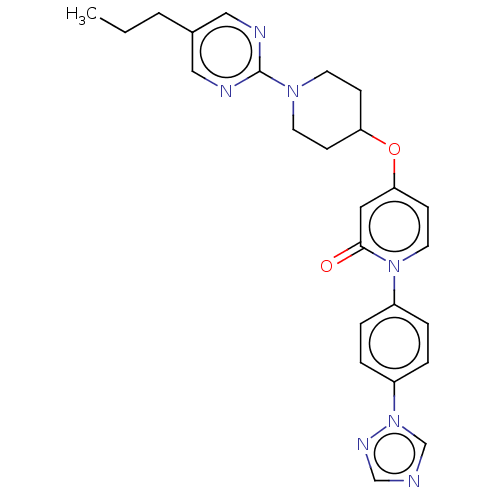
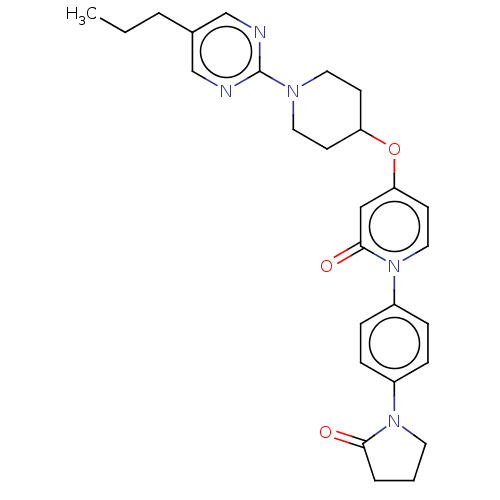
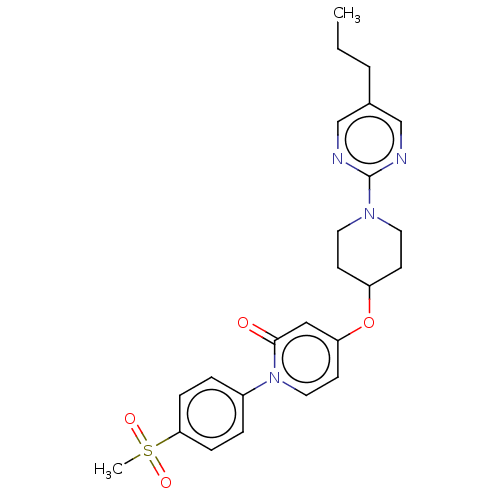
Affinity DataIC50: 122nMAssay Description:Displacement of fluorescent labeled derivative from recombinant human hepatic glucokinase incubated for 30 mins in presence of 12 mM glucose by fluor...More data for this Ligand-Target Pair
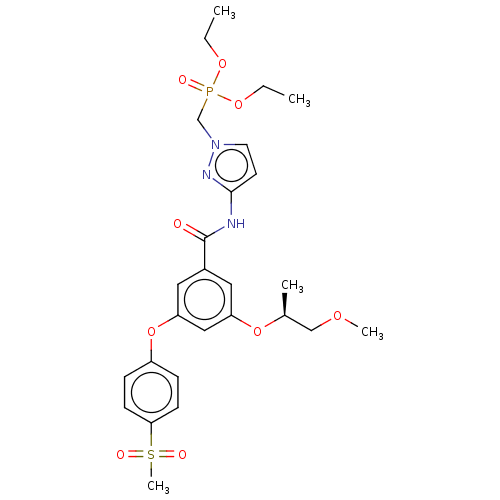
Affinity DataIC50: 123nMAssay Description:Displacement of fluorescent labeled derivative from recombinant human hepatic glucokinase incubated for 30 mins in presence of 12 mM glucose by fluor...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
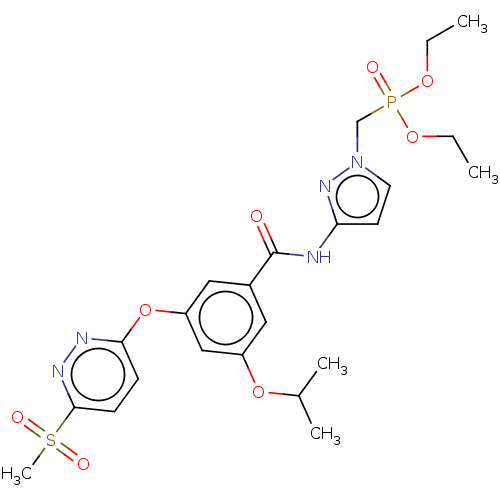
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of human ERG by patch clamp assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.70E+3nMAssay Description:Inhibition of CYP2C8 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2B6 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: 3.30E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: 4.40E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+4nMAssay Description:Inhibition of P-gp transporter (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: >8.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: >8.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: >8.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: >8.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: >8.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: >8.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: >8.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: >8.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: >8.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: >8.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: >8.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: >8.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: >8.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: >8.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: >8.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: >8.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: >8.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: >8.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: >8.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataIC50: >8.00E+4nMAssay Description:Inhibition of hERG by patch clamp methodMore data for this Ligand-Target Pair
TargetGlucose-dependent insulinotropic receptor(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
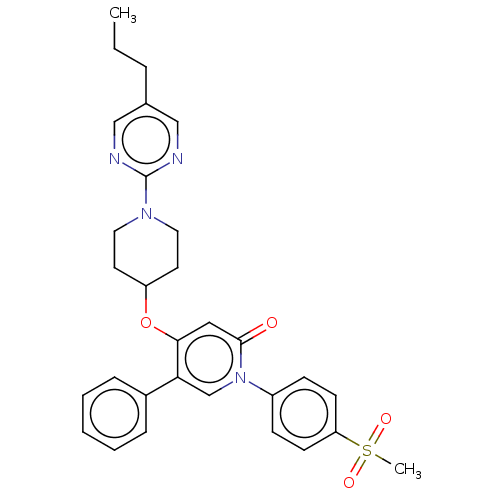
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at human GPR119More data for this Ligand-Target Pair
TargetGlucose-dependent insulinotropic receptor(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataEC50: 31nMAssay Description:Agonist activity at human GPR119More data for this Ligand-Target Pair
TargetGlucose-dependent insulinotropic receptor(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at human GPR119More data for this Ligand-Target Pair
TargetGlucose-dependent insulinotropic receptor(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at human GPR119More data for this Ligand-Target Pair
TargetGlucose-dependent insulinotropic receptor(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataEC50: 9nMAssay Description:Agonist activity at human GPR119More data for this Ligand-Target Pair
TargetGlucose-dependent insulinotropic receptor(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataEC50: 18nMAssay Description:Agonist activity at human GPR119More data for this Ligand-Target Pair
TargetGlucose-dependent insulinotropic receptor(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataEC50: 95nMAssay Description:Agonist activity at human GPR119More data for this Ligand-Target Pair
TargetGlucose-dependent insulinotropic receptor(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataEC50: 63nMAssay Description:Agonist activity at human GPR119More data for this Ligand-Target Pair
TargetGlucose-dependent insulinotropic receptor(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataEC50: 32nMAssay Description:Agonist activity at human GPR119More data for this Ligand-Target Pair
TargetGlucose-dependent insulinotropic receptor(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataEC50: 37nMAssay Description:Agonist activity at human GPR119More data for this Ligand-Target Pair
TargetGlucose-dependent insulinotropic receptor(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataEC50: 11nMAssay Description:Agonist activity at human GPR119More data for this Ligand-Target Pair
TargetGlucose-dependent insulinotropic receptor(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataEC50: 41nMAssay Description:Agonist activity at human GPR119More data for this Ligand-Target Pair
TargetGlucose-dependent insulinotropic receptor(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataEC50: 16nMAssay Description:Agonist activity at human GPR119More data for this Ligand-Target Pair
TargetGlucose-dependent insulinotropic receptor(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataEC50: 23nMAssay Description:Agonist activity at human GPR119More data for this Ligand-Target Pair
TargetGlucose-dependent insulinotropic receptor(Homo sapiens (Human))
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Departments Of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical And Translational Research, And Pharmaceutical Candi
Curated by ChEMBL
Affinity DataEC50: 117nMAssay Description:Agonist activity at human GPR119More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)