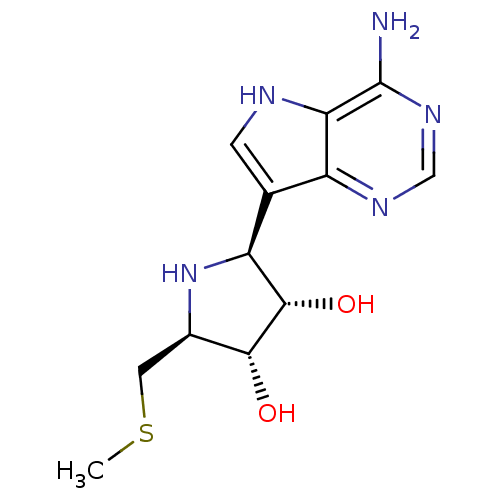
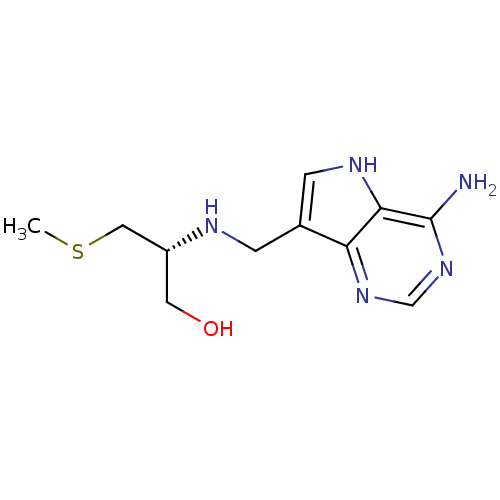
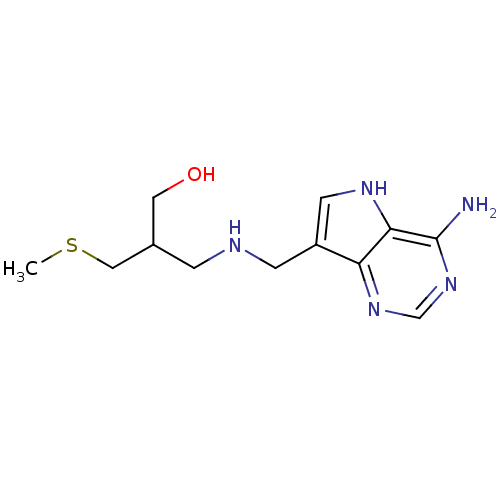
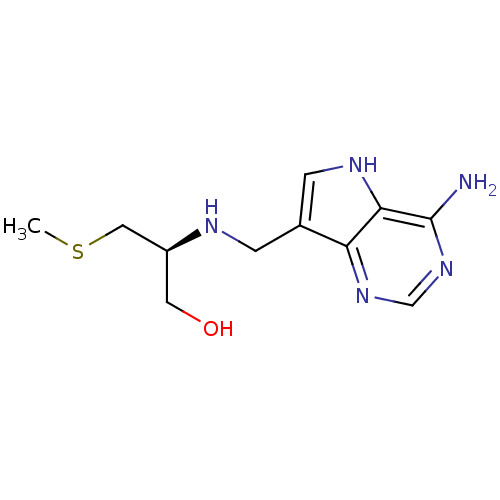
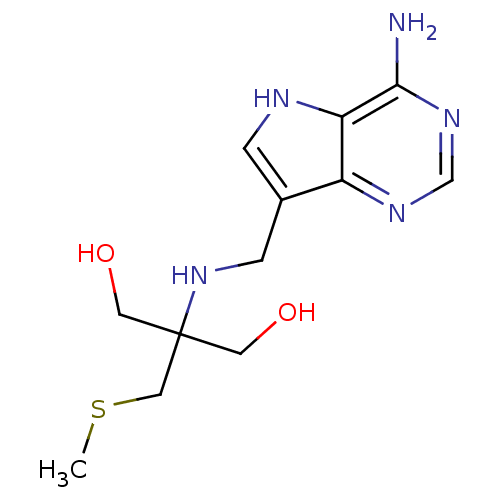
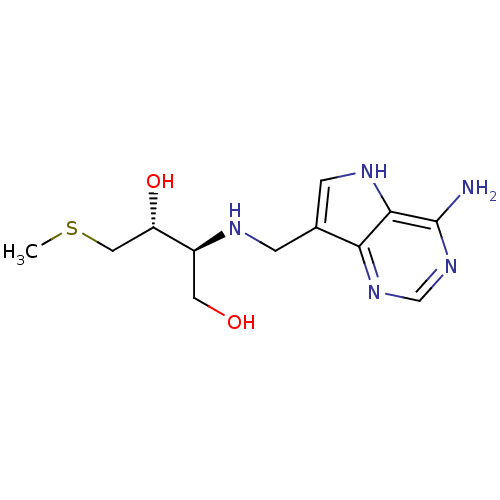
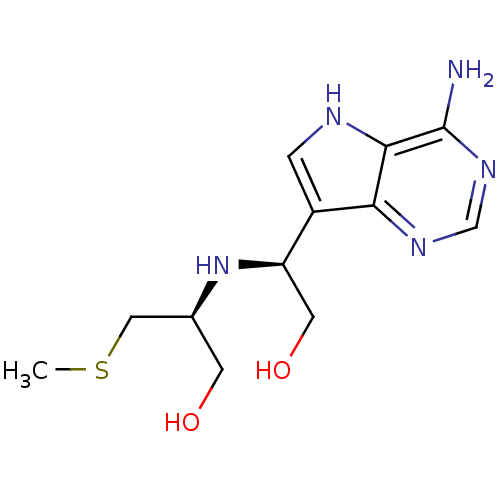
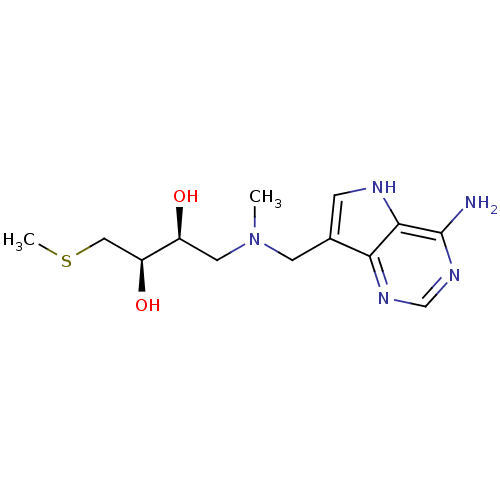
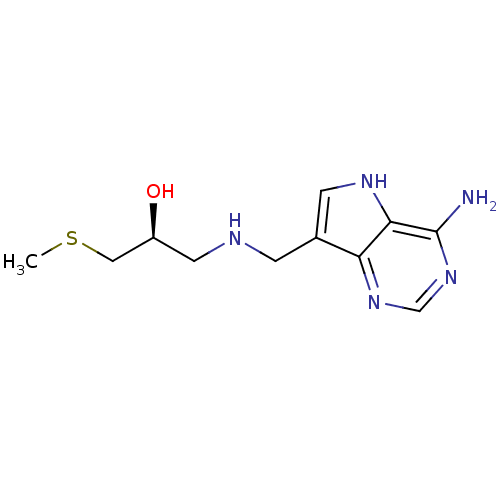
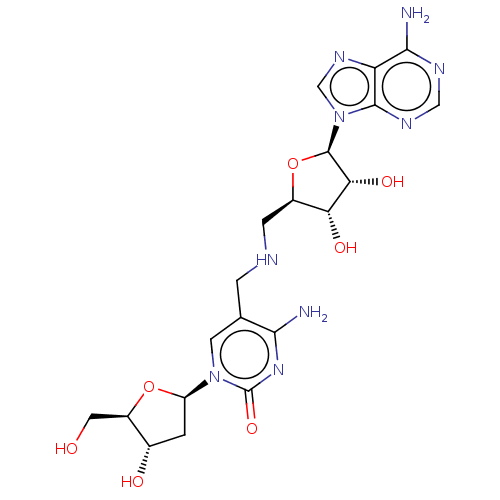
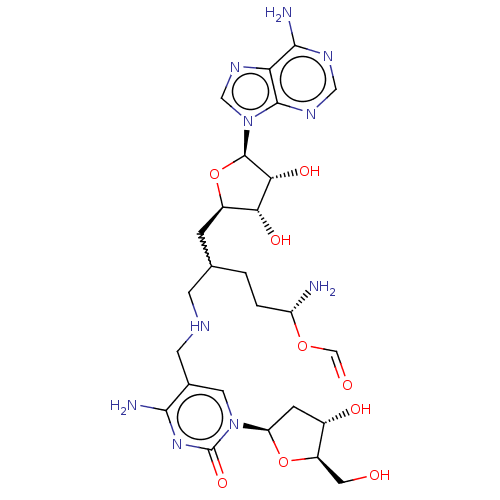
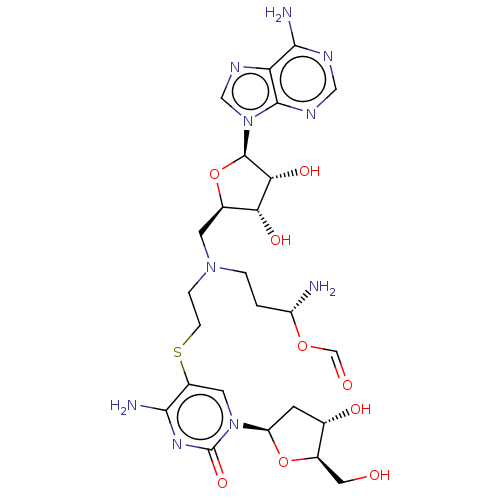
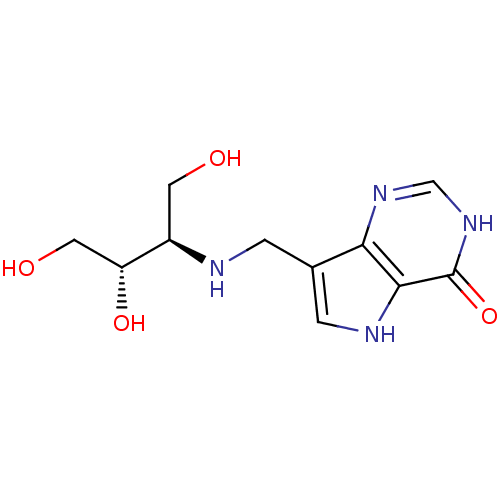
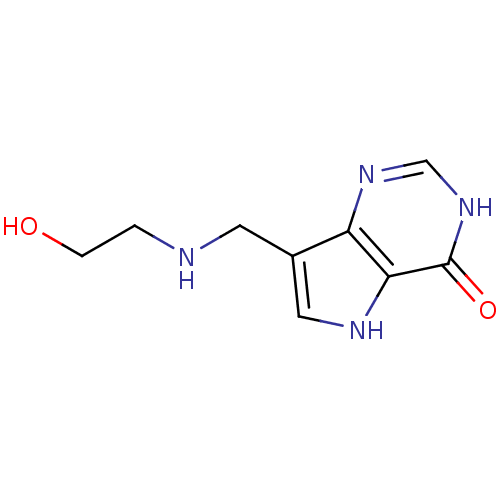
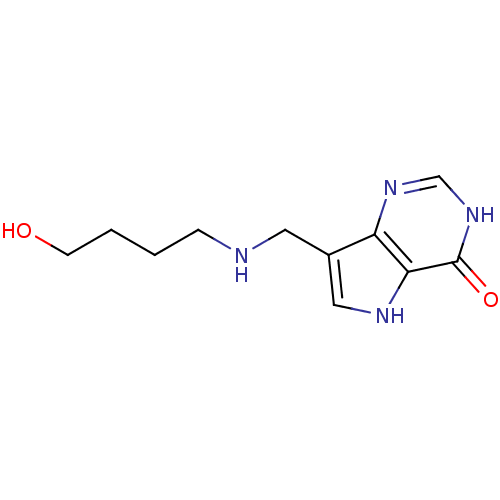
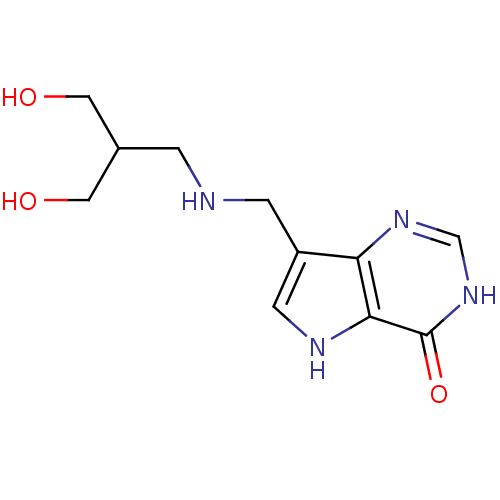
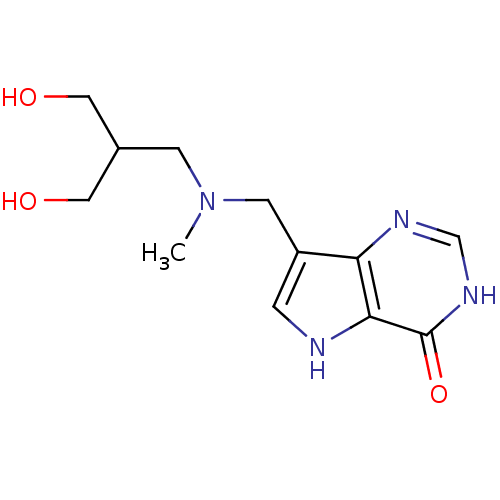
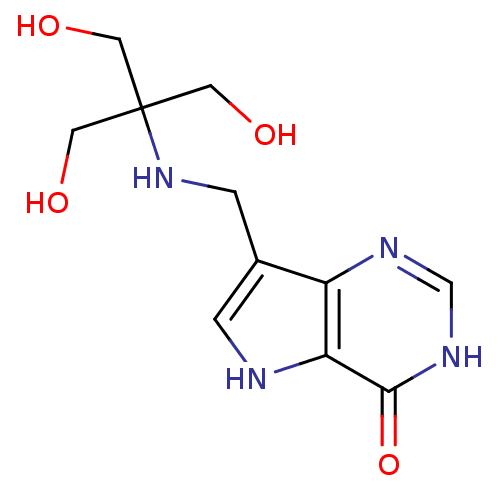
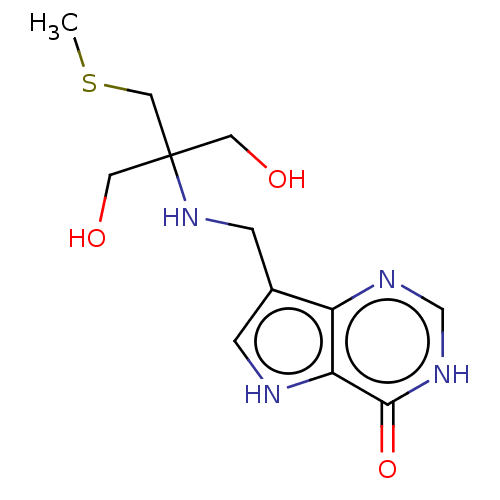
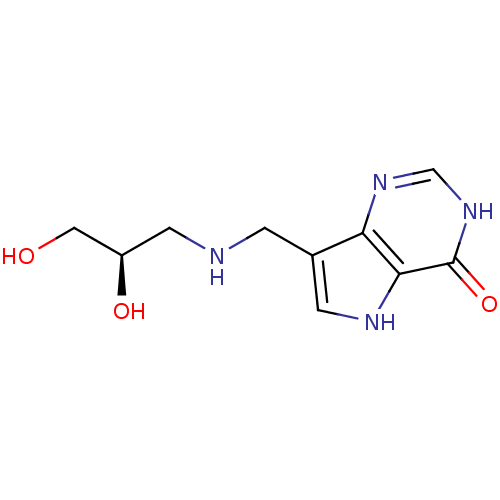
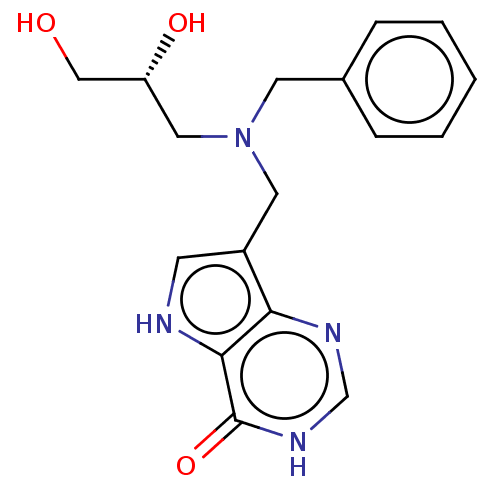
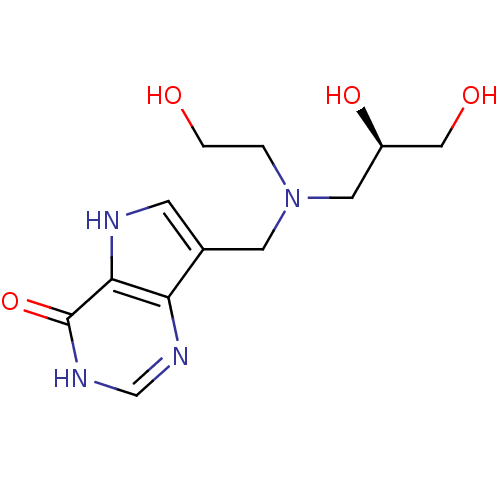
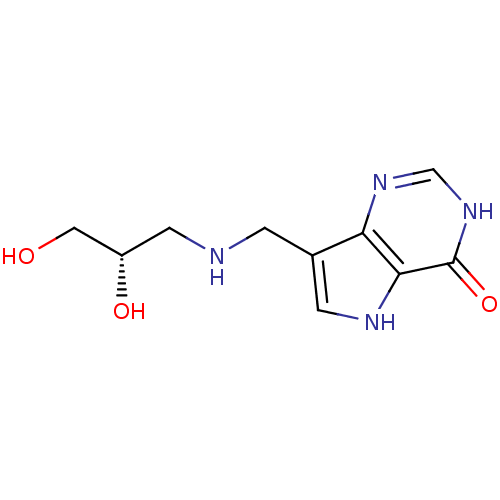
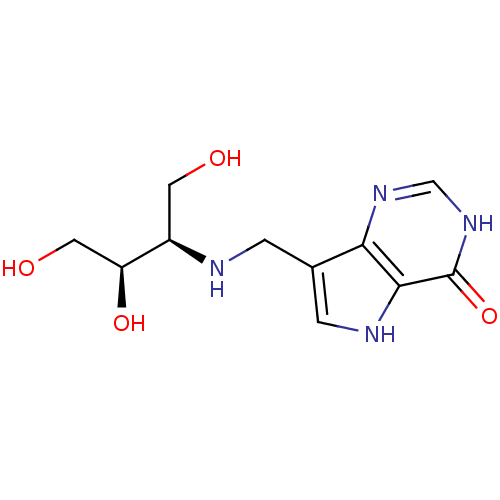
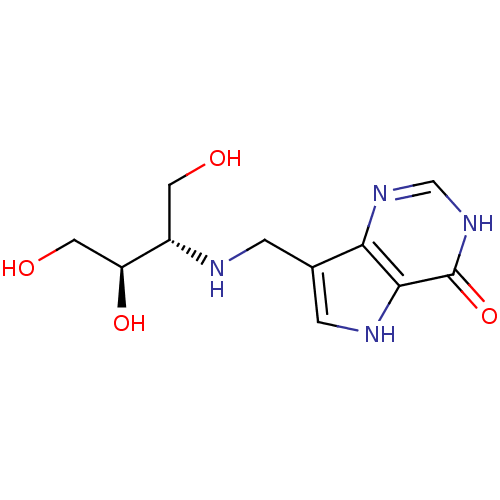
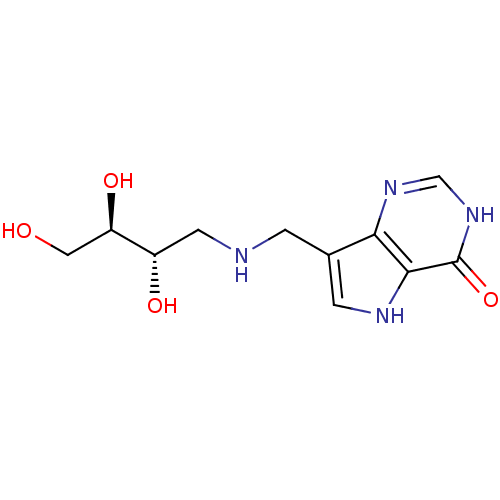
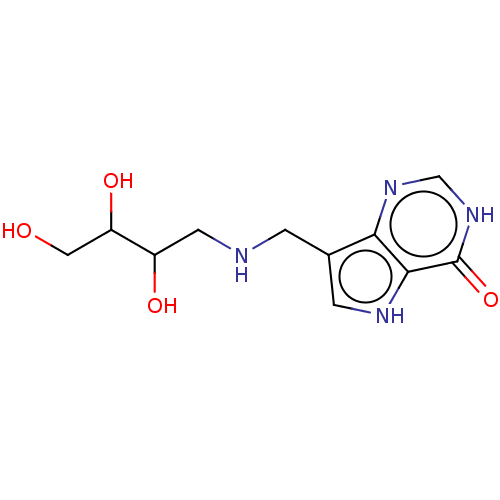
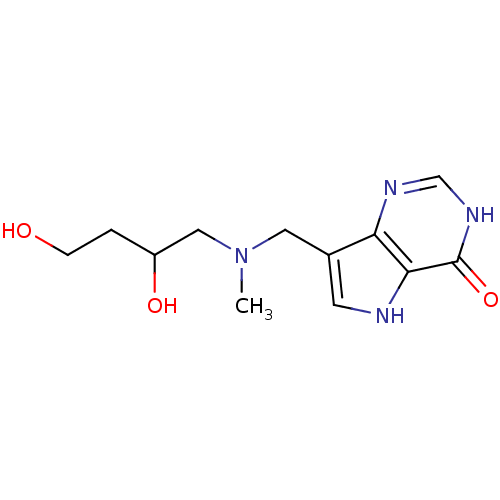
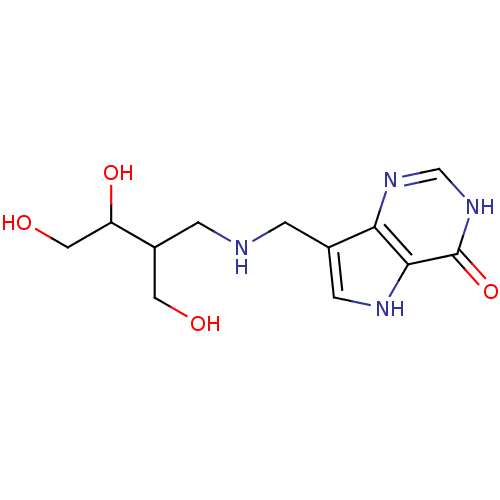
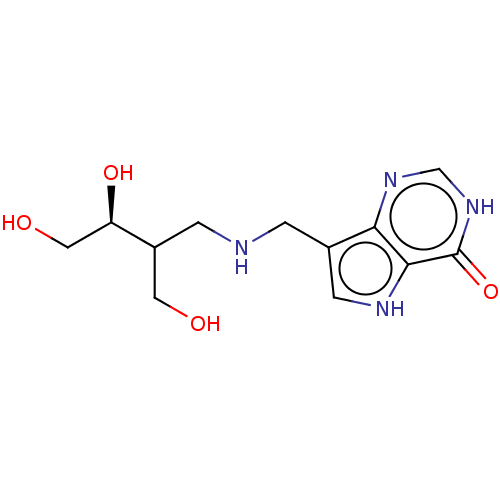
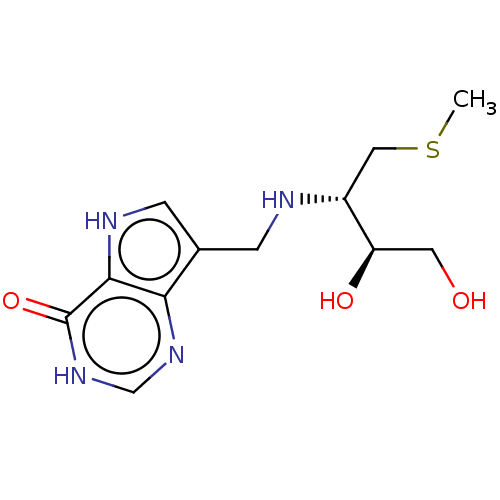
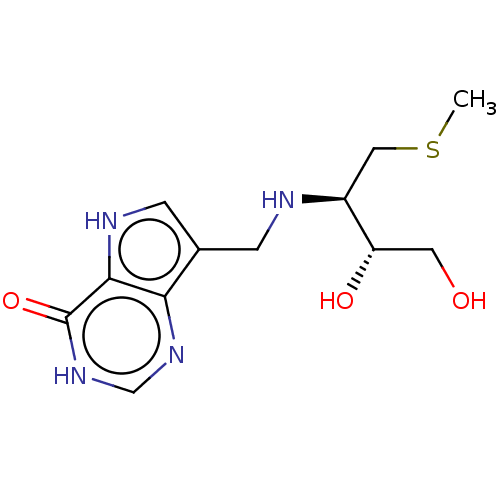
TargetS-methyl-5'-thioadenosine phosphorylase(Homo sapiens (Human))
Industrial Research
Curated by ChEMBL
Industrial Research
Curated by ChEMBL
Affinity DataKi: 0.0900nMAssay Description:Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysisMore data for this Ligand-Target Pair
TargetS-methyl-5'-thioadenosine phosphorylase(Homo sapiens (Human))
Industrial Research
Curated by ChEMBL
Industrial Research
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysisMore data for this Ligand-Target Pair
TargetS-methyl-5'-thioadenosine phosphorylase(Homo sapiens (Human))
Industrial Research
Curated by ChEMBL
Industrial Research
Curated by ChEMBL
Affinity DataKi: 4.40nMAssay Description:Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysisMore data for this Ligand-Target Pair
TargetS-methyl-5'-thioadenosine phosphorylase(Homo sapiens (Human))
Industrial Research
Curated by ChEMBL
Industrial Research
Curated by ChEMBL
Affinity DataKi: 5.20nMAssay Description:Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysisMore data for this Ligand-Target Pair
TargetS-methyl-5'-thioadenosine phosphorylase(Homo sapiens (Human))
Industrial Research
Curated by ChEMBL
Industrial Research
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysisMore data for this Ligand-Target Pair
TargetS-methyl-5'-thioadenosine phosphorylase(Homo sapiens (Human))
Industrial Research
Curated by ChEMBL
Industrial Research
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysisMore data for this Ligand-Target Pair
TargetS-methyl-5'-thioadenosine phosphorylase(Homo sapiens (Human))
Industrial Research
Curated by ChEMBL
Industrial Research
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysisMore data for this Ligand-Target Pair
TargetS-methyl-5'-thioadenosine phosphorylase(Homo sapiens (Human))
Industrial Research
Curated by ChEMBL
Industrial Research
Curated by ChEMBL
Affinity DataKi: 34nMAssay Description:Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysisMore data for this Ligand-Target Pair
TargetS-methyl-5'-thioadenosine phosphorylase(Homo sapiens (Human))
Industrial Research
Curated by ChEMBL
Industrial Research
Curated by ChEMBL
Affinity DataKi: 34nMAssay Description:Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysisMore data for this Ligand-Target Pair
TargetS-methyl-5'-thioadenosine phosphorylase(Homo sapiens (Human))
Industrial Research
Curated by ChEMBL
Industrial Research
Curated by ChEMBL
Affinity DataKi: 34nMAssay Description:Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysisMore data for this Ligand-Target Pair
TargetS-methyl-5'-thioadenosine phosphorylase(Homo sapiens (Human))
Industrial Research
Curated by ChEMBL
Industrial Research
Curated by ChEMBL
Affinity DataKi: 34nMAssay Description:Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysisMore data for this Ligand-Target Pair
TargetS-methyl-5'-thioadenosine phosphorylase(Homo sapiens (Human))
Industrial Research
Curated by ChEMBL
Industrial Research
Curated by ChEMBL
Affinity DataKi: 87nMAssay Description:Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysisMore data for this Ligand-Target Pair
TargetS-methyl-5'-thioadenosine phosphorylase(Homo sapiens (Human))
Industrial Research
Curated by ChEMBL
Industrial Research
Curated by ChEMBL
Affinity DataKi: 87nMAssay Description:Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysisMore data for this Ligand-Target Pair
TargetS-methyl-5'-thioadenosine phosphorylase(Homo sapiens (Human))
Industrial Research
Curated by ChEMBL
Industrial Research
Curated by ChEMBL
Affinity DataKi: 105nMAssay Description:Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysisMore data for this Ligand-Target Pair
TargetS-methyl-5'-thioadenosine phosphorylase(Homo sapiens (Human))
Industrial Research
Curated by ChEMBL
Industrial Research
Curated by ChEMBL
Affinity DataKi: 130nMAssay Description:Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysisMore data for this Ligand-Target Pair
TargetS-methyl-5'-thioadenosine phosphorylase(Homo sapiens (Human))
Industrial Research
Curated by ChEMBL
Industrial Research
Curated by ChEMBL
Affinity DataKi: 368nMAssay Description:Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysisMore data for this Ligand-Target Pair
TargetS-methyl-5'-thioadenosine phosphorylase(Homo sapiens (Human))
Industrial Research
Curated by ChEMBL
Industrial Research
Curated by ChEMBL
Affinity DataKi: 602nMAssay Description:Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysisMore data for this Ligand-Target Pair
Affinity DataIC50: 700nMAssay Description:The DNMT1 assays were carried to determine the IC50 with transition state analogs. The reaction (100 μL) containing 50 mM Tris-HCl pH 8.0, 100 m...More data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:The DNMT1 assays were carried to determine the IC50 with transition state analogs. The reaction (100 μL) containing 50 mM Tris-HCl pH 8.0, 100 m...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:The DNMT1 assays were carried to determine the IC50 with transition state analogs. The reaction (100 μL) containing 50 mM Tris-HCl pH 8.0, 100 m...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:The DNMT1 assays were carried to determine the IC50 with transition state analogs. The reaction (100 μL) containing 50 mM Tris-HCl pH 8.0, 100 m...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:The DNMT1 assays were carried to determine the IC50 with transition state analogs. The reaction (100 μL) containing 50 mM Tris-HCl pH 8.0, 100 m...More data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:The DNMT1 assays were carried to determine the IC50 with transition state analogs. The reaction (100 μL) containing 50 mM Tris-HCl pH 8.0, 100 m...More data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:The DNMT1 assays were carried to determine the IC50 with transition state analogs. The reaction (100 μL) containing 50 mM Tris-HCl pH 8.0, 100 m...More data for this Ligand-Target Pair
Affinity DataIC50: 1.54E+4nMAssay Description:The DNMT1 assays were carried to determine the IC50 with transition state analogs. The reaction (100 μL) containing 50 mM Tris-HCl pH 8.0, 100 m...More data for this Ligand-Target Pair
Affinity DataKd: 0.00860nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 0.469nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 1.10nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 25nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 14.1nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 3.70nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 0.620nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 3nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 14.9nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 300nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 165nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 4.20nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 96nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 5.20nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 4.30nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 1nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 31nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 84nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 227nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 0.780nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 900nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 15nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 74nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 71nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKd: 142nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)