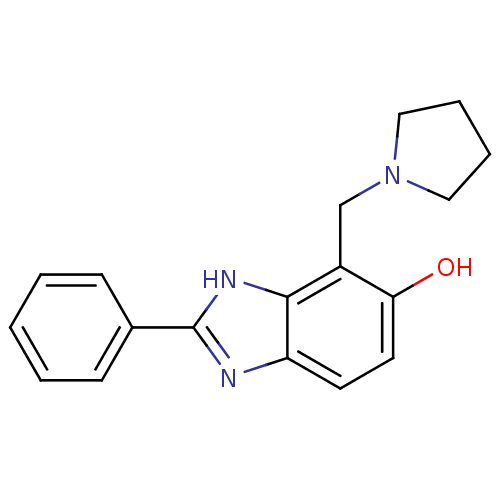
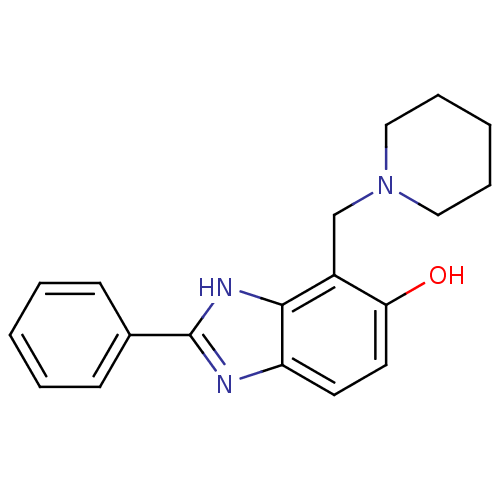
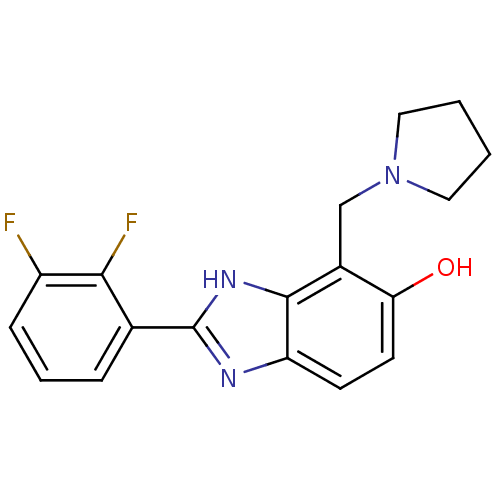
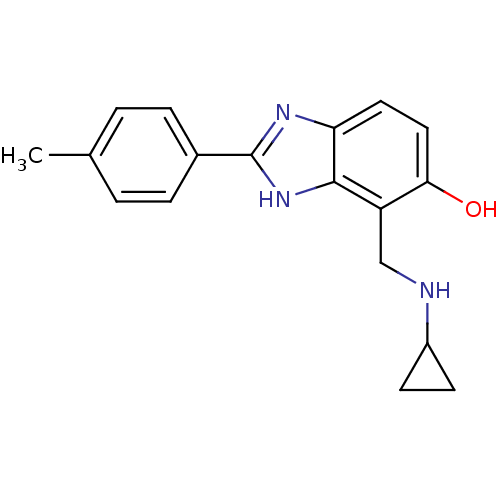
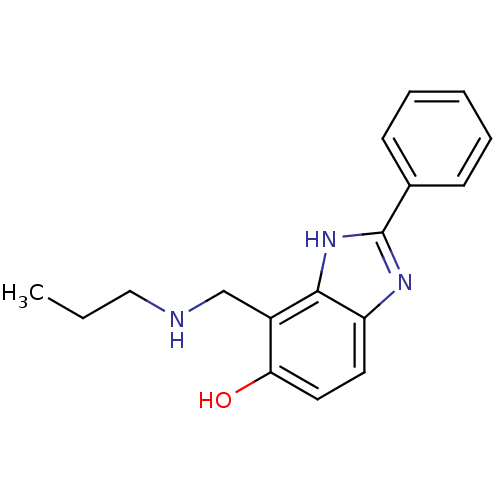
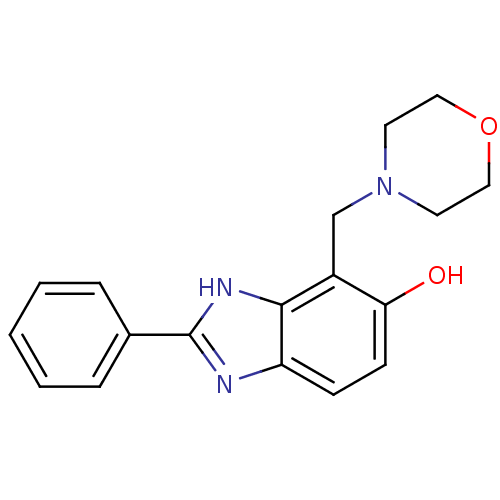
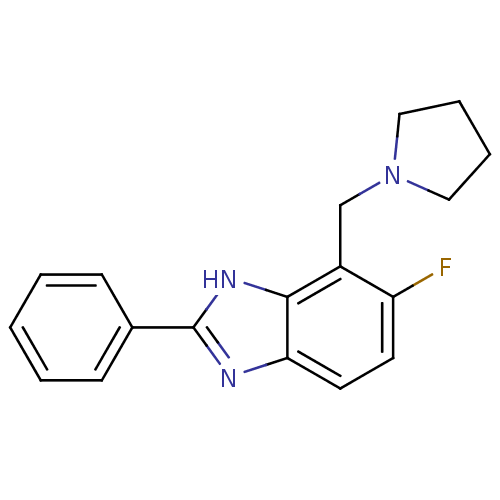
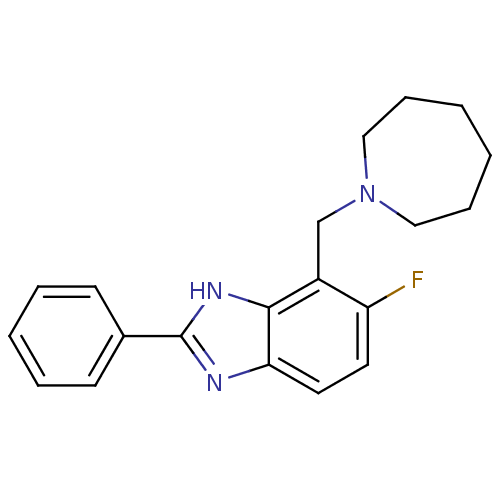
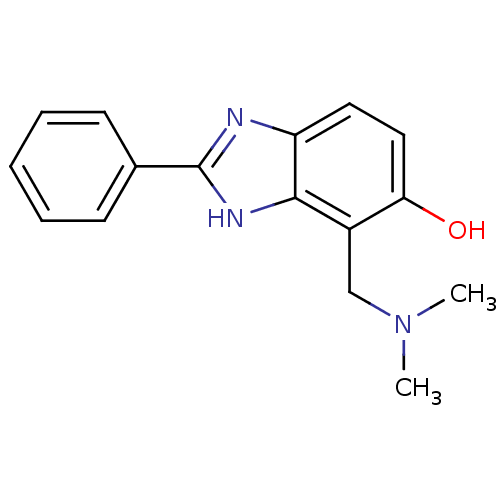
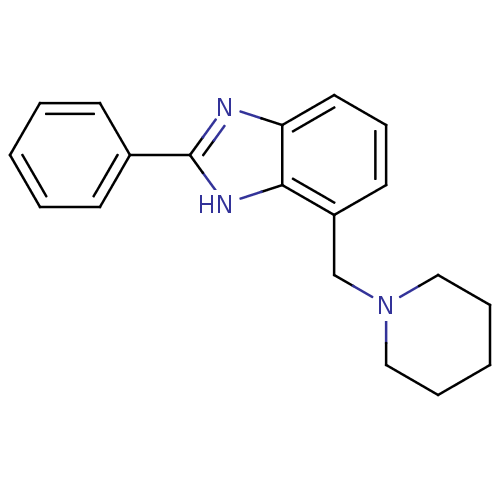
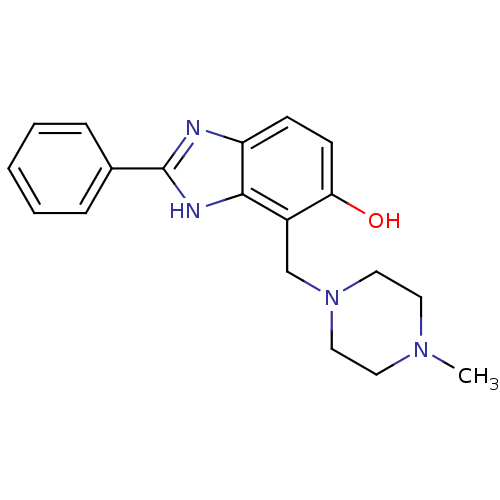
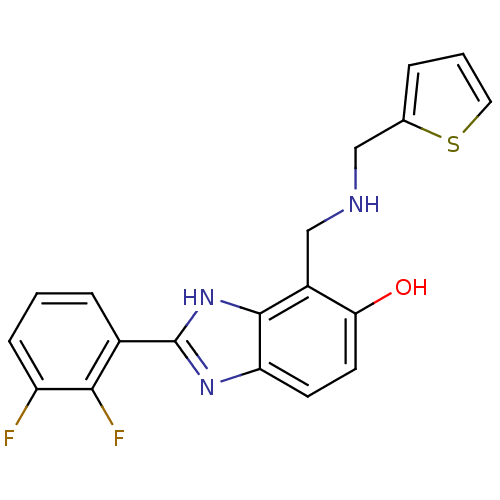
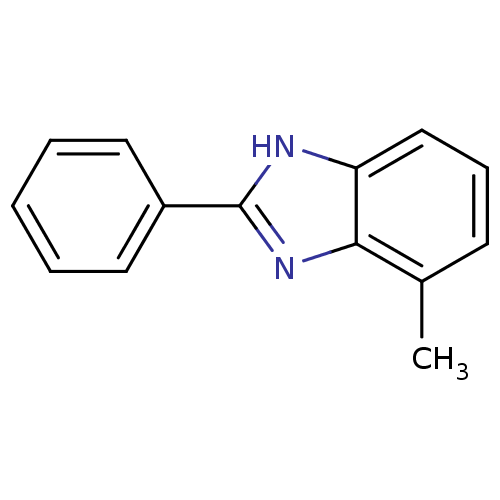
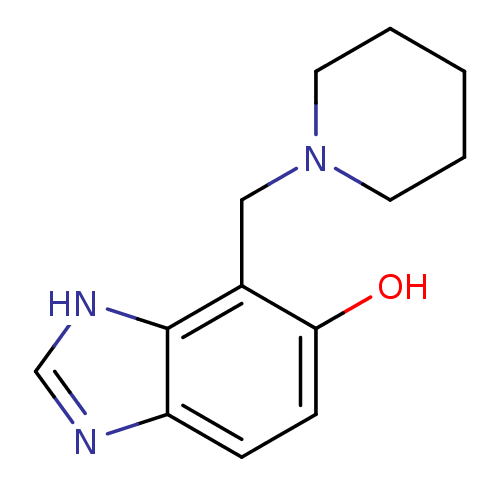
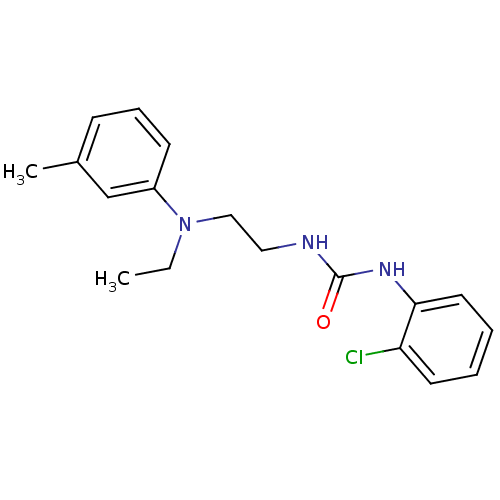
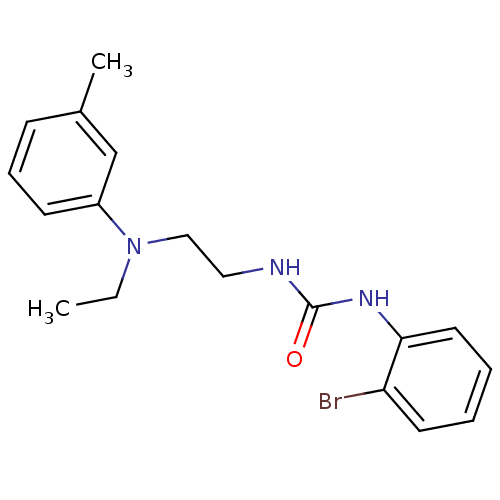
Affinity DataIC50: 7.94nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 7.94nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 7.94nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 9nMpH: 5.3Assay Description:Antagonist activity at human TRPV1 expressed in HEK293 cells assessed as inhibition of pH 5.3 acid-induced calcium influx by whole cell patch clamp a...More data for this Ligand-Target Pair
Affinity DataIC50: 12.6nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 31.6nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 39.8nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 39.8nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 39.8nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 40nMpH: 5.3Assay Description:Antagonist activity at human TRPV1 expressed in HEK293 cells assessed as inhibition of pH 5.3 acid-induced calcium influx by FLIPR assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 49nMAssay Description:Antagonist activity at human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced calcium influx by whole cell patch clamp ass...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Cavia porcellus)
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 63nMpH: 5.3Assay Description:Antagonist activity at guinea pig TRPV1 expressed in HEK293 cells assessed as inhibition of pH 5.3 acid-induced calcium influx by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 79.4nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 79.4nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 79.4nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rattus norvegicus (rat))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 100nMpH: 5.3Assay Description:Antagonist activity at rat TRPV1 expressed in HEK293 cells assessed as inhibition of pH 5.3 acid-induced calcium influx by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 251nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 398nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 398nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 631nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 794nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 794nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.58E+3nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 2.51E+3nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Displacement of [3H]-Ro256981 from human NR2B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+4nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataEC50: 63nMpH: 7.2 T: 2°CAssay Description:One day before the assay was performed, human TRPV1 expressed in 1321N1 astrocytoma cells were plated onto 96-well assay plates and grown until 2.5 h...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataEC50: 126nMpH: 7.2 T: 2°CAssay Description:One day before the assay was performed, human TRPV1 expressed in 1321N1 astrocytoma cells were plated onto 96-well assay plates and grown until 2.5 h...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataEC50: 158nMpH: 7.2 T: 2°CAssay Description:One day before the assay was performed, human TRPV1 expressed in 1321N1 astrocytoma cells were plated onto 96-well assay plates and grown until 2.5 h...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataEC50: 20nMpH: 7.2 T: 2°CAssay Description:One day before the assay was performed, human TRPV1 expressed in 1321N1 astrocytoma cells were plated onto 96-well assay plates and grown until 2.5 h...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataEC50: 16nMpH: 7.2 T: 2°CAssay Description:One day before the assay was performed, human TRPV1 expressed in 1321N1 astrocytoma cells were plated onto 96-well assay plates and grown until 2.5 h...More data for this Ligand-Target Pair