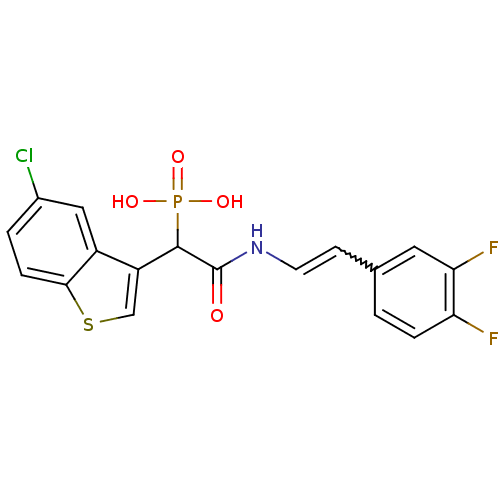
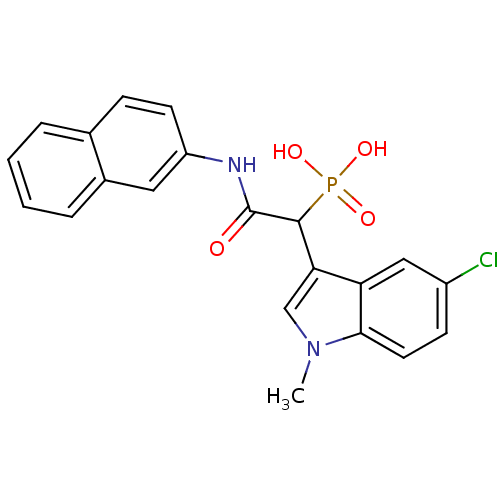
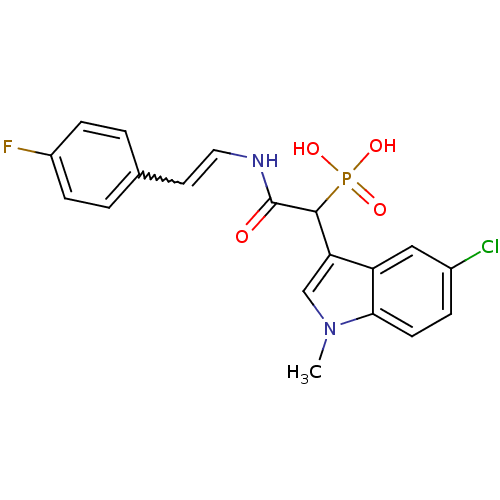
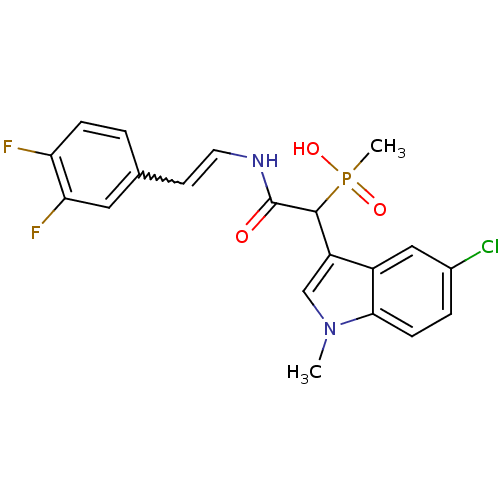
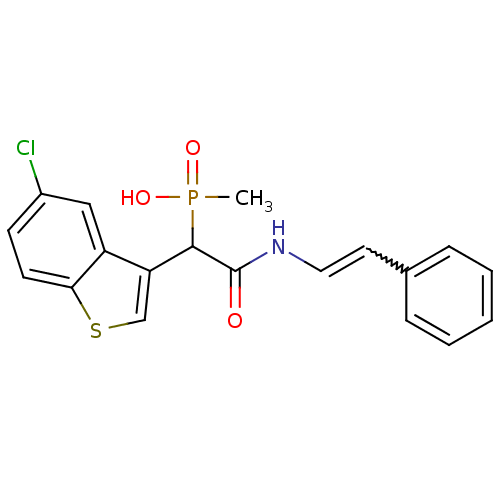
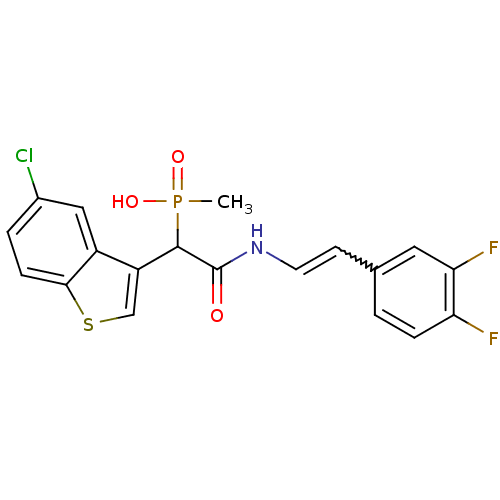
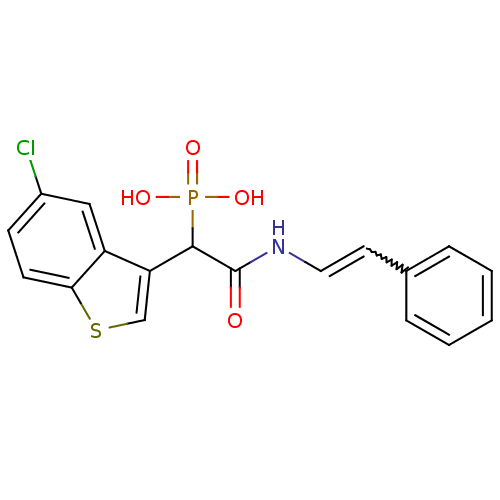
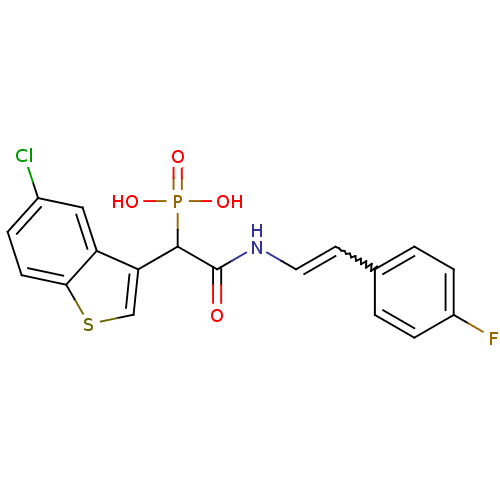
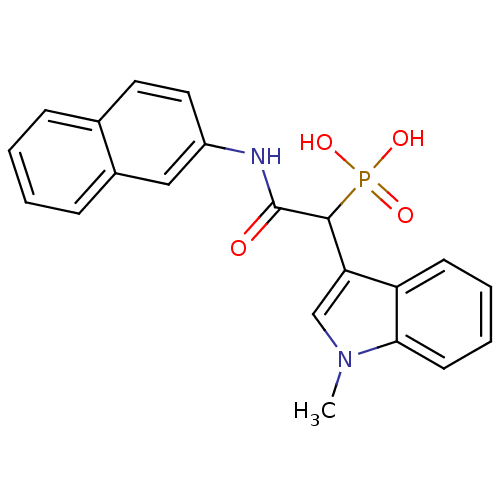
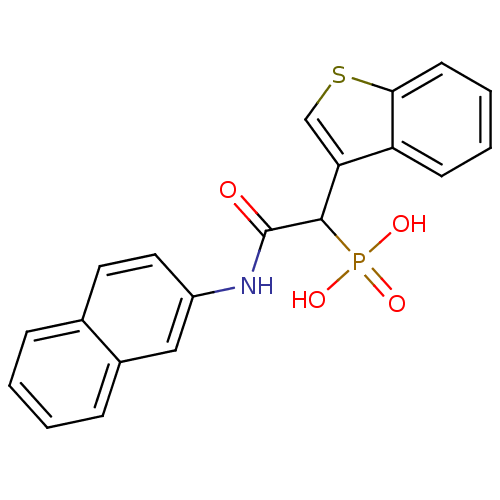
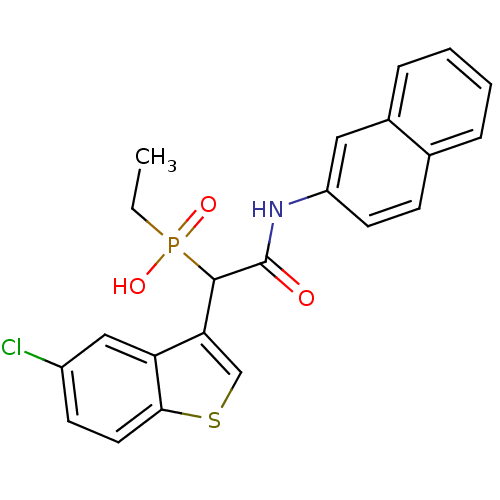
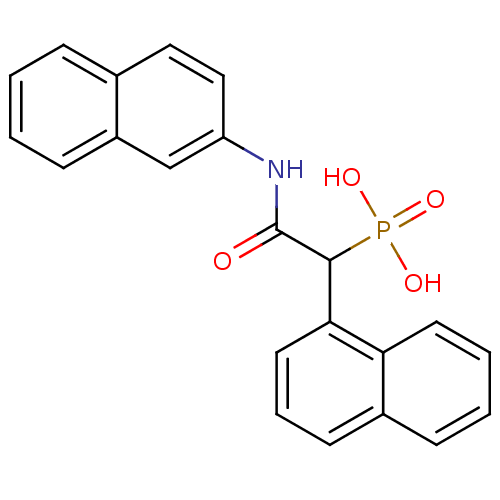
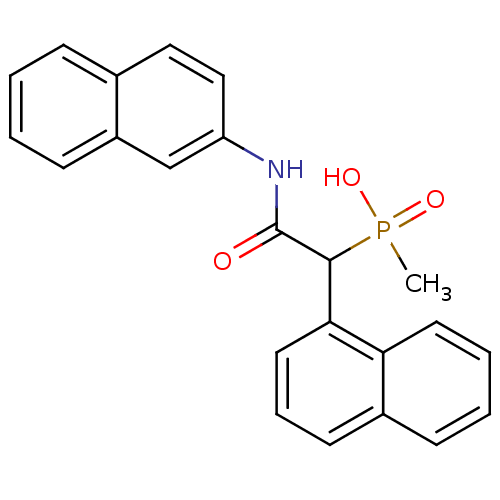
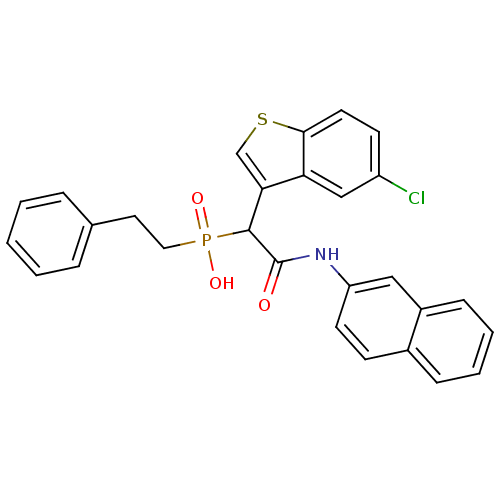
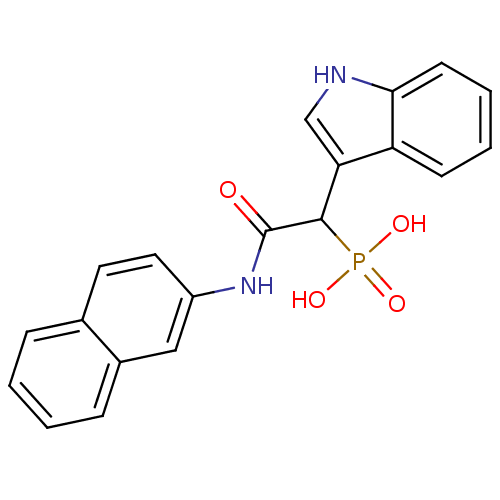
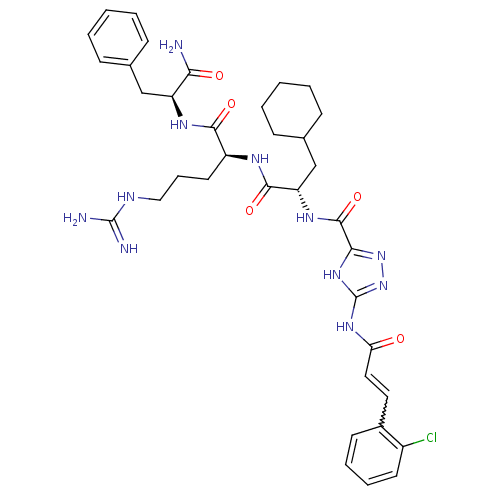
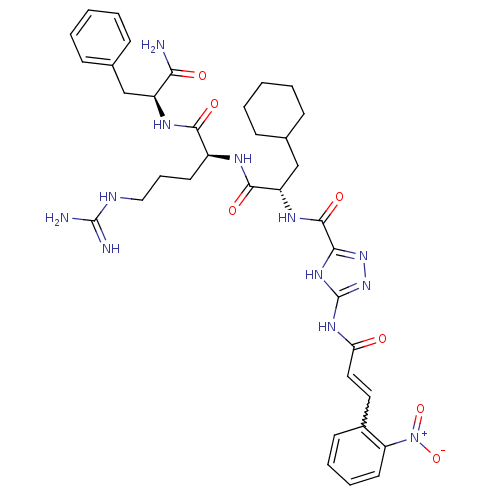
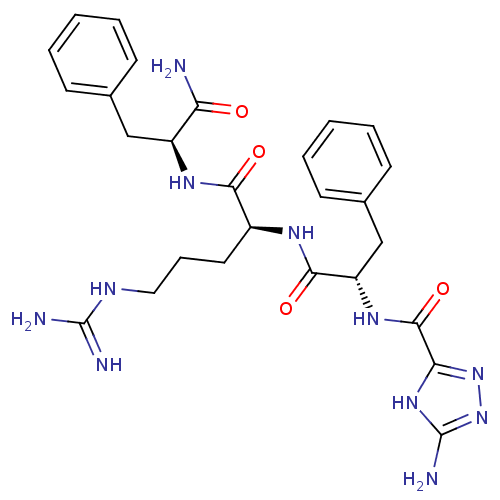
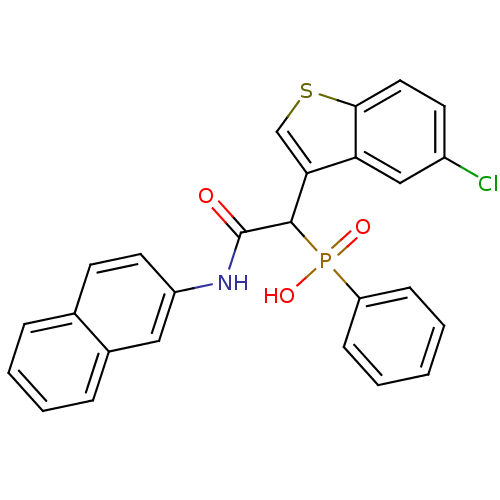
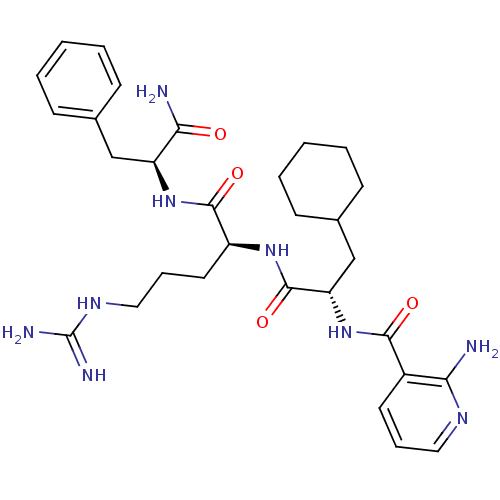
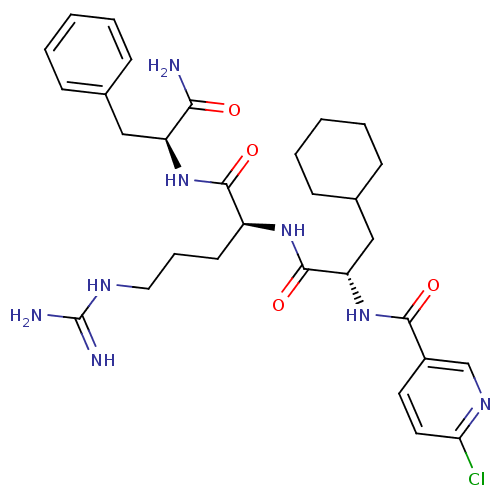
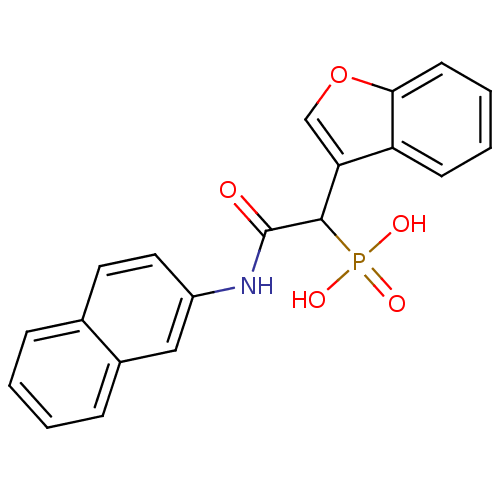
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 2.30nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 36nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetCathepsin G(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 38nMAssay Description:Inhibition of human neutrophil Cat GMore data for this Ligand-Target Pair
TargetCathepsin G(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 9.50E+3nMAssay Description:Inhibition of human neutrophil Cat GMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 3.5nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 4.5nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 21nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 29nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 58nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 66nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 90nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 160nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 165nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 190nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 240nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 280nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 550nMAssay Description:Activation of human platelet aggregation (gel-filtered platelets) induced by alpha thrombinMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 920nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to Thrombin receptor 1 (PAR-1) on membranes from CHRF cellsMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Activation of human platelet aggregation (gel-filtered platelets) induced by alpha thrombinMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to Thrombin receptor 1 (PAR-1) on membranes from CHRF cellsMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to thrombin receptor (PAR-1) on membranes from CHRF cellsMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to thrombin receptor (PAR-1) on membranes from CHRF cellsMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to thrombin receptor (PAR-1) on membranes from CHRF cellsMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.70E+3nMAssay Description:Activation of human platelet aggregation (gel-filtered platelets) induced by alpha thrombinMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to thrombin receptor (PAR-1) on membranes from CHRF cellsMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to thrombin receptor (PAR-1) on membranes from CHRF cellsMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Activation of human platelet aggregation (gel-filtered platelets) induced by alpha thrombinMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to thrombin receptor (PAR-1) on membranes from CHRF cellsMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to thrombin receptor (PAR-1) on membranes from CHRF cellsMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to thrombin receptor (PAR-1) on membranes from CHRF cellsMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to thrombin receptor (PAR-1) on membranes from CHRF cellsMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to Thrombin receptor 1 (PAR-1) on membranes from CHRF cellsMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to Thrombin receptor 1 (PAR-1) on membranes from CHRF cellsMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.40E+3nMAssay Description:Activation of human platelet aggregation (gel-filtered platelets) induced by alpha thrombinMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to Thrombin receptor 1 (PAR-1) on membranes from CHRF cellsMore data for this Ligand-Target Pair
TargetProteinase-activated receptor 1(Homo sapiens (Human))
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
The R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.90E+3nMAssay Description:Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to thrombin receptor (PAR-1) on membranes from CHRF cellsMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)