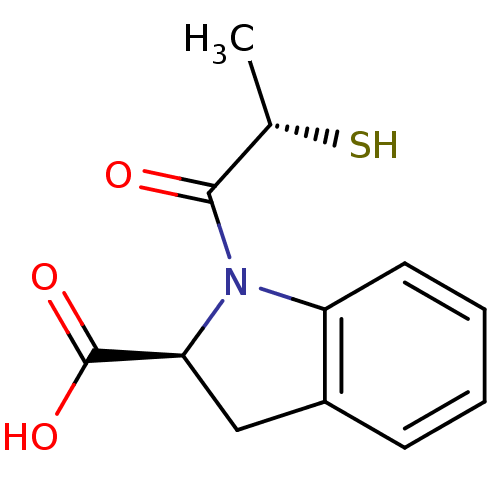
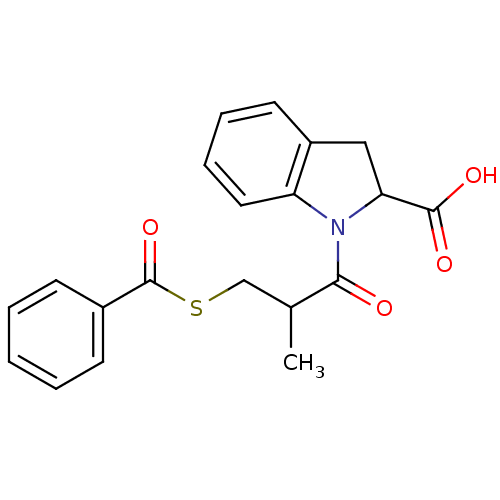
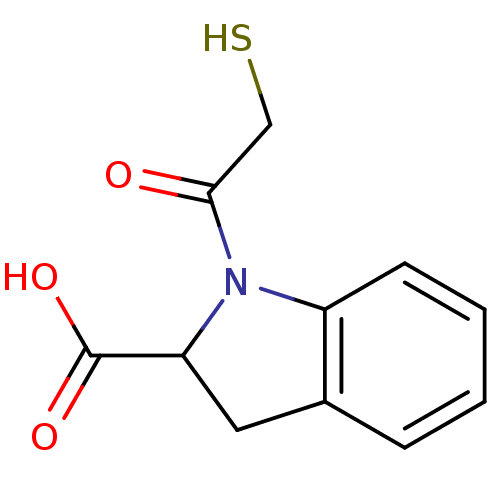
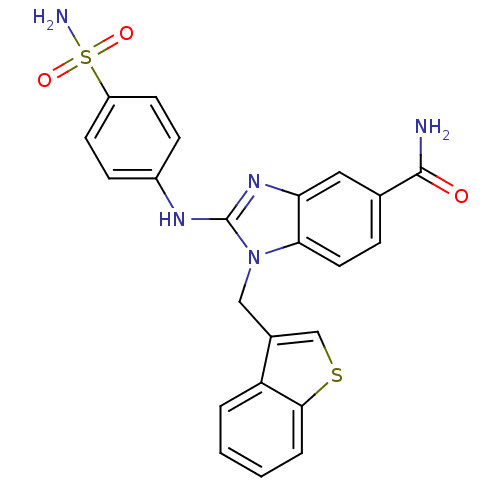
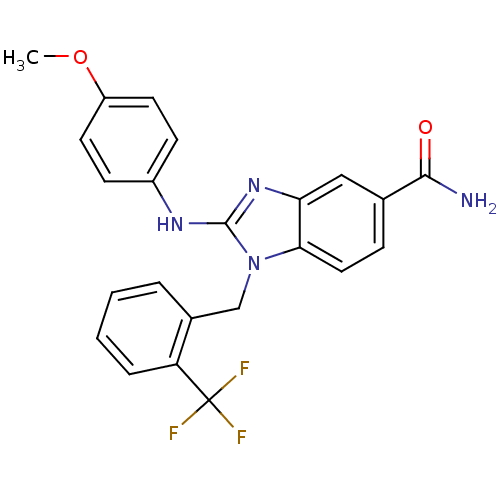
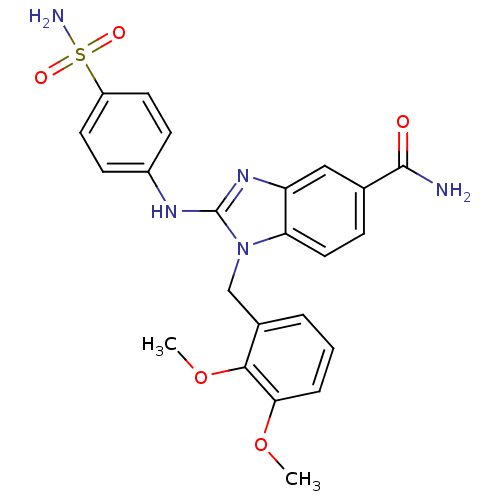
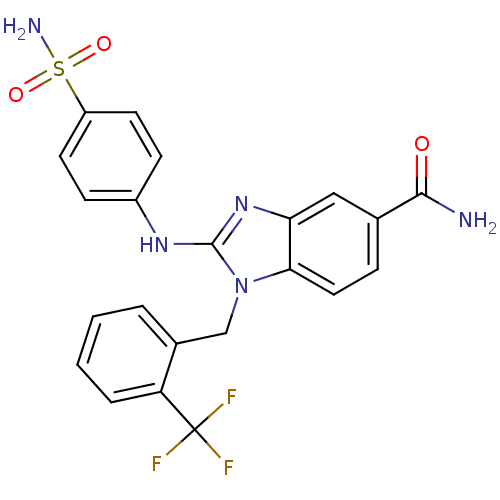
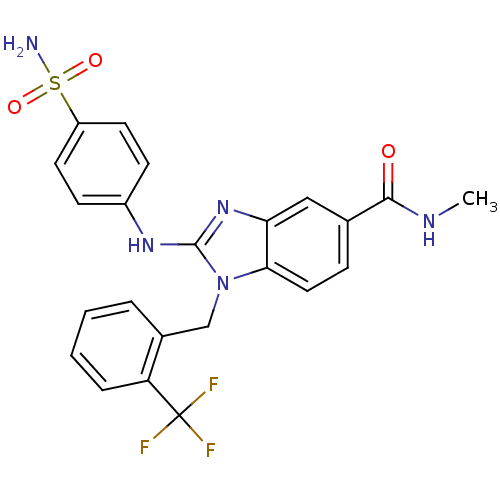
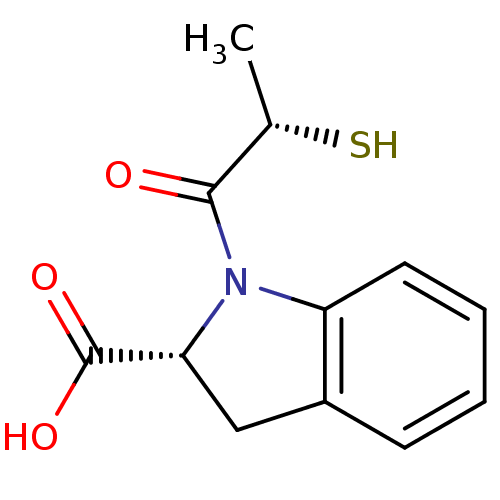
Affinity DataIC50: 3.70nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 59nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 74nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 78nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 94nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 97nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 140nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 320nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 380nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 410nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetKinesin-like protein KIF11(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 520nMAssay Description:Inhibition of human kinesin spindle protein by endpoint assayMore data for this Ligand-Target Pair
Affinity DataIC50: 540nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetKinesin-like protein KIF11(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of human kinesin spindle protein by endpoint assayMore data for this Ligand-Target Pair
TargetKinesin-like protein KIF11(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of human kinesin spindle protein by endpoint assayMore data for this Ligand-Target Pair
TargetKinesin-like protein KIF11(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of human kinesin spindle protein by endpoint assayMore data for this Ligand-Target Pair
TargetKinesin-like protein KIF11(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of human kinesin spindle protein by endpoint assayMore data for this Ligand-Target Pair
TargetKinesin-like protein KIF11(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of human kinesin spindle protein by endpoint assayMore data for this Ligand-Target Pair
TargetKinesin-like protein KIF11(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of human kinesin spindle protein by endpoint assayMore data for this Ligand-Target Pair
TargetKinesin-like protein KIF11(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human kinesin spindle protein by endpoint assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.50E+3nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+3nMAssay Description:Inhibition of CYP2C9 preincubated with compoundMore data for this Ligand-Target Pair
TargetKinesin-like protein KIF11(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.80E+3nMAssay Description:Inhibition of human kinesin spindle protein by endpoint assayMore data for this Ligand-Target Pair
TargetKinesin-like protein KIF11(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of human kinesin spindle protein by endpoint assayMore data for this Ligand-Target Pair
TargetKinesin-like protein KIF11(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.20E+3nMAssay Description:Inhibition of human kinesin spindle protein by endpoint assayMore data for this Ligand-Target Pair
TargetKinesin-like protein KIF11(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of human kinesin spindle protein by endpoint assayMore data for this Ligand-Target Pair
TargetKinesin-like protein KIF11(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 6.90E+3nMAssay Description:Inhibition of human kinesin spindle protein by endpoint assayMore data for this Ligand-Target Pair
Affinity DataIC50: >7.00E+3nMAssay Description:Inhibition of CYP2C9 preincubated with compoundMore data for this Ligand-Target Pair
TargetKinesin-like protein KIF11(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 7.40E+3nMAssay Description:Inhibition of human kinesin spindle protein by endpoint assayMore data for this Ligand-Target Pair
TargetKinesin-like protein KIF11(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 7.90E+3nMAssay Description:Inhibition of human kinesin spindle protein by endpoint assayMore data for this Ligand-Target Pair
Affinity DataIC50: >8.00E+3nMAssay Description:Inhibition of CYP2C9 preincubated with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of CYP2C9 coincubated with compoundMore data for this Ligand-Target Pair
TargetKinesin-like protein KIF11(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 9.20E+3nMAssay Description:Inhibition of human kinesin spindle protein by endpoint assayMore data for this Ligand-Target Pair
TargetKinesin-like protein KIF11(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.34E+4nMAssay Description:Inhibition of human kinesin spindle protein by endpoint assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibition of CYP2C9 coincubated with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+4nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: >1.50E+4nMAssay Description:Inhibition of CYP2D6 preincubated with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+4nMAssay Description:In vitro inhibitory activity against Angiotensin I converting enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: >1.50E+4nMAssay Description:Inhibition of CYP2D6 preincubated with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of CYP2C9 coincubated with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: >1.50E+4nMAssay Description:Inhibition of CYP3A4 preincubated with compoundMore data for this Ligand-Target Pair
Affinity DataIC50: >1.50E+4nMAssay Description:Inhibition of CYP2D6 preincubated with compoundMore data for this Ligand-Target Pair