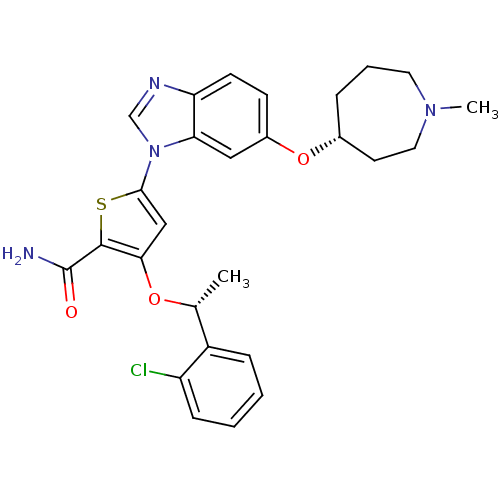
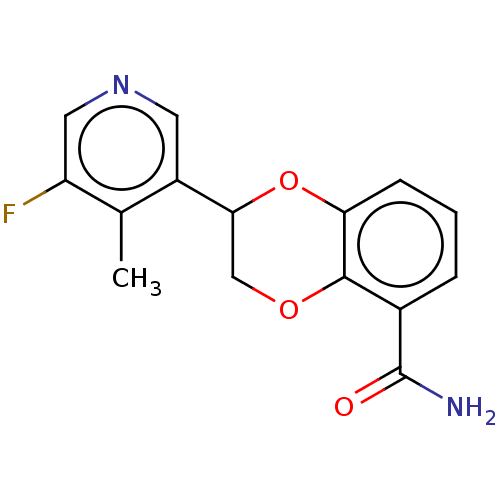
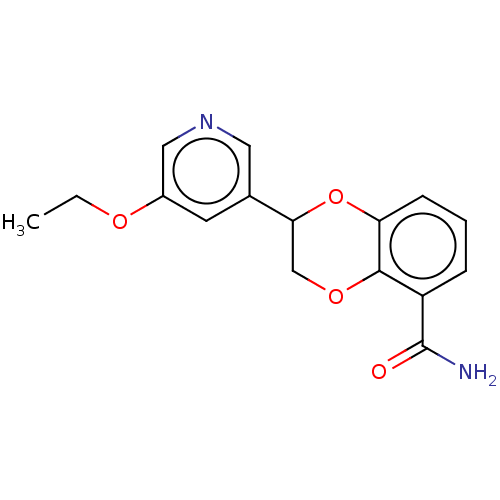
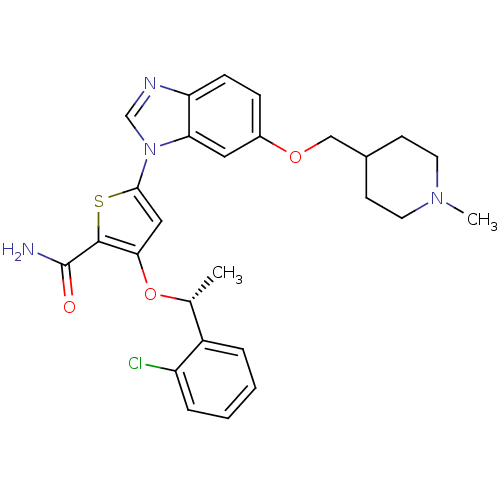
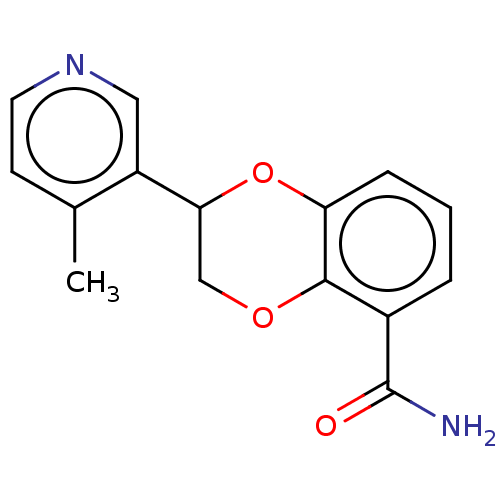
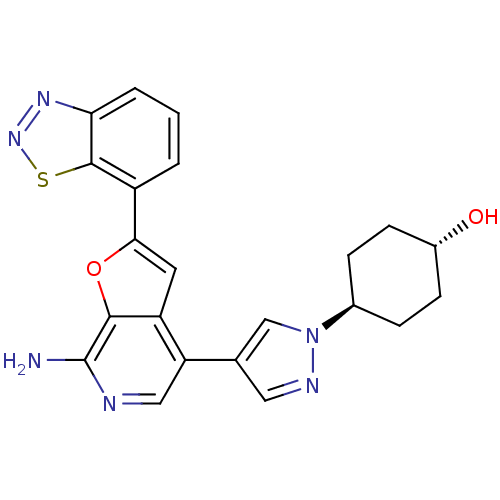
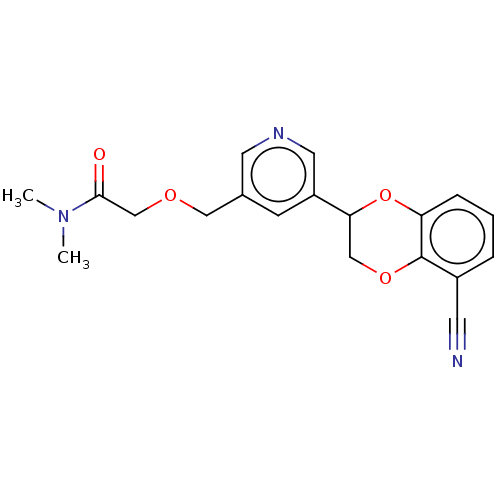
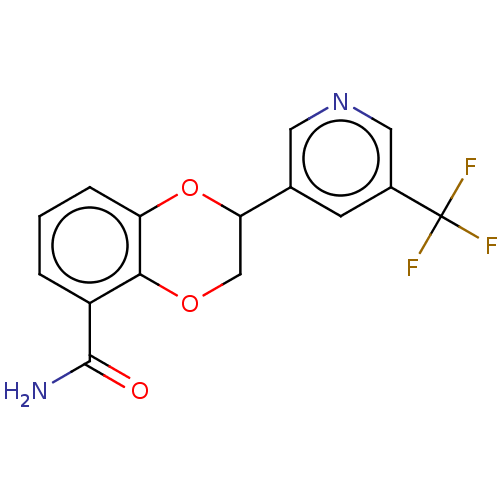
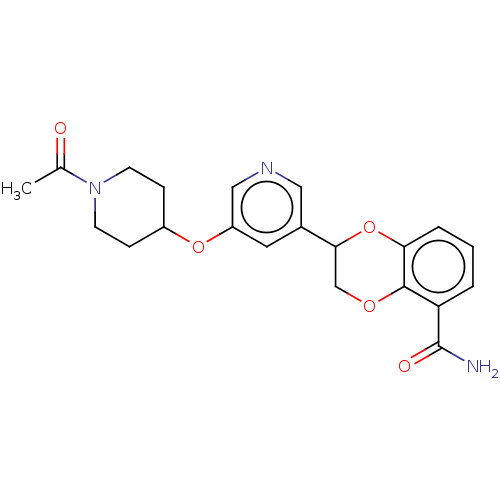
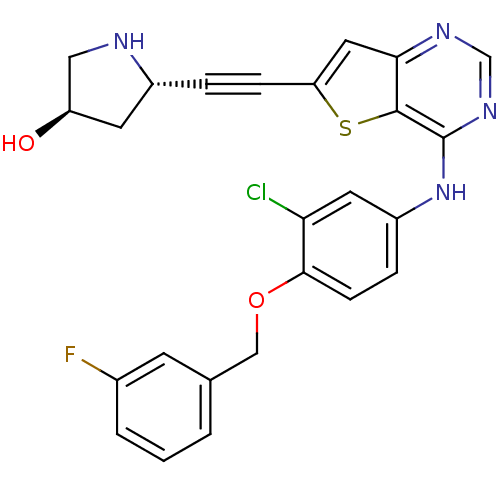
Affinity DataIC50: 0.800nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1(Homo sapiens (Human))
Osi Pharmaceuticals
Curated by ChEMBL
Osi Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of truncated TAK1-TAB1(unknown origin) using MKK7 as substrate by ALPHAScreen assay in presence of ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-...More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Boehringer Ingelheim International
US Patent
Boehringer Ingelheim International
US Patent
Affinity DataIC50: 4nMAssay Description:Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi...More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Boehringer Ingelheim International
US Patent
Boehringer Ingelheim International
US Patent
Affinity DataIC50: 5nMAssay Description:Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi...More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Boehringer Ingelheim International
US Patent
Boehringer Ingelheim International
US Patent
Affinity DataIC50: 5nMAssay Description:Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi...More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Boehringer Ingelheim International
US Patent
Boehringer Ingelheim International
US Patent
Affinity DataIC50: 6nMAssay Description:Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of EGFR kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-...More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Boehringer Ingelheim International
US Patent
Boehringer Ingelheim International
US Patent
Affinity DataIC50: 7.5nMAssay Description:Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi...More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Boehringer Ingelheim International
US Patent
Boehringer Ingelheim International
US Patent
Affinity DataIC50: 8nMAssay Description:Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi...More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Boehringer Ingelheim International
US Patent
Boehringer Ingelheim International
US Patent
Affinity DataIC50: 8nMAssay Description:Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi...More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Boehringer Ingelheim International
US Patent
Boehringer Ingelheim International
US Patent
Affinity DataIC50: 8nMAssay Description:Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi...More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Boehringer Ingelheim International
US Patent
Boehringer Ingelheim International
US Patent
Affinity DataIC50: 9nMAssay Description:Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMpH: 7.2 T: 2°CAssay Description:The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1(Homo sapiens (Human))
Osi Pharmaceuticals
Curated by ChEMBL
Osi Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of TAK1-TAB1 (unknown origin) by alphascreen assay in presence of ATPMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Boehringer Ingelheim International
US Patent
Boehringer Ingelheim International
US Patent
Affinity DataIC50: 9nMAssay Description:Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi...More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Boehringer Ingelheim International
US Patent
Boehringer Ingelheim International
US Patent
Affinity DataIC50: 10nMAssay Description:Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi...More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Boehringer Ingelheim International
US Patent
Boehringer Ingelheim International
US Patent
Affinity DataIC50: 10nMAssay Description:Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 7(Homo sapiens (Human))
Osi Pharmaceuticals
Curated by ChEMBL
Osi Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of TAK1 in human HCT116 cells assessed as inhibition of TNF-alpha-stimulated JNK phosphorylationMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Boehringer Ingelheim International
US Patent
Boehringer Ingelheim International
US Patent
Affinity DataIC50: 10nMAssay Description:Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi...More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Boehringer Ingelheim International
US Patent
Boehringer Ingelheim International
US Patent
Affinity DataIC50: 10nMAssay Description:Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi...More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Boehringer Ingelheim International
US Patent
Boehringer Ingelheim International
US Patent
Affinity DataIC50: 11nMAssay Description:Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of ErbB2 kinaseMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Boehringer Ingelheim International
US Patent
Boehringer Ingelheim International
US Patent
Affinity DataIC50: 11nMAssay Description:Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi...More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Boehringer Ingelheim International
US Patent
Boehringer Ingelheim International
US Patent
Affinity DataIC50: 11nMAssay Description:Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 7(Homo sapiens (Human))
Osi Pharmaceuticals
Curated by ChEMBL
Osi Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of TAK1 in human HCT116 cells assessed as inhibition of TNF-alpha-stimulated JNK phosphorylationMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Boehringer Ingelheim International
US Patent
Boehringer Ingelheim International
US Patent
Affinity DataIC50: 12nMAssay Description:Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1(Homo sapiens (Human))
Osi Pharmaceuticals
Curated by ChEMBL
Osi Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of truncated TAK1-TAB1(unknown origin) using MKK7 as substrate by ALPHAScreen assay in presence of ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of ErbB2 kinaseMore data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Boehringer Ingelheim International
US Patent
Boehringer Ingelheim International
US Patent
Affinity DataIC50: 13nMAssay Description:Assays are performed in 96-well format in a final volume of 60 μL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additi...More data for this Ligand-Target Pair




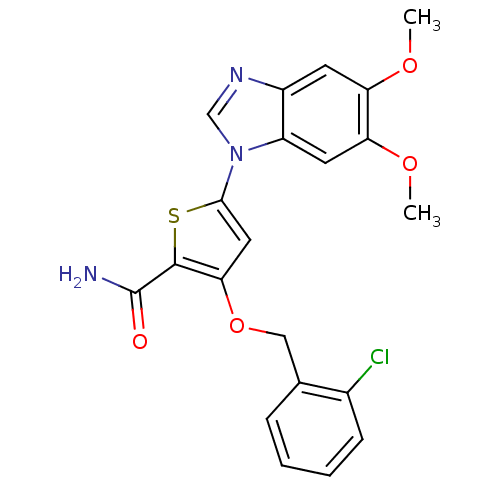

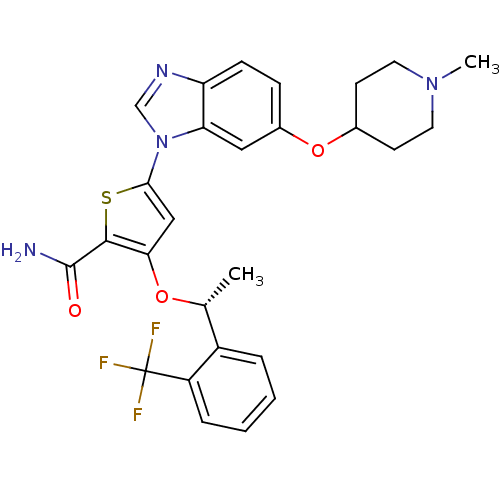
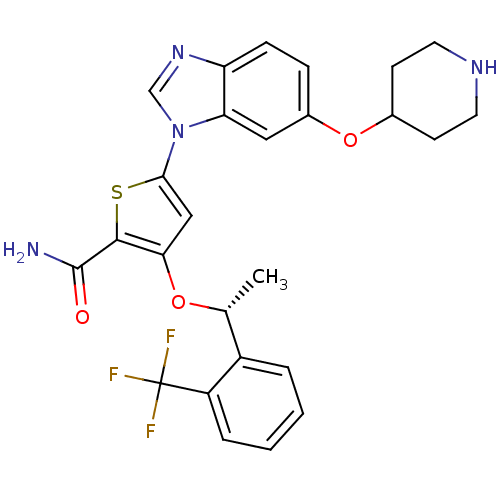
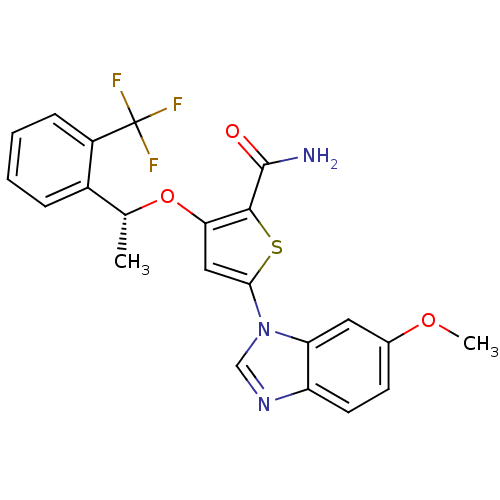
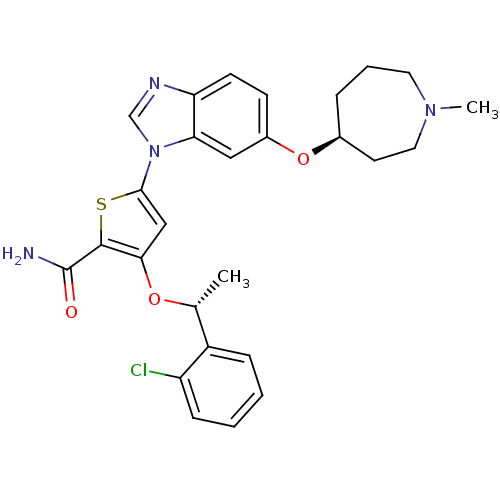

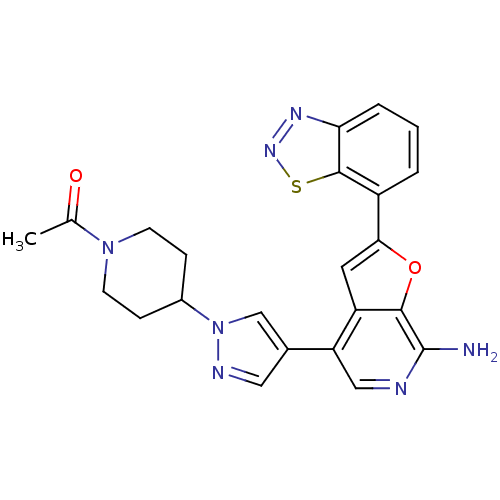
 3D Structure (crystal)
3D Structure (crystal)