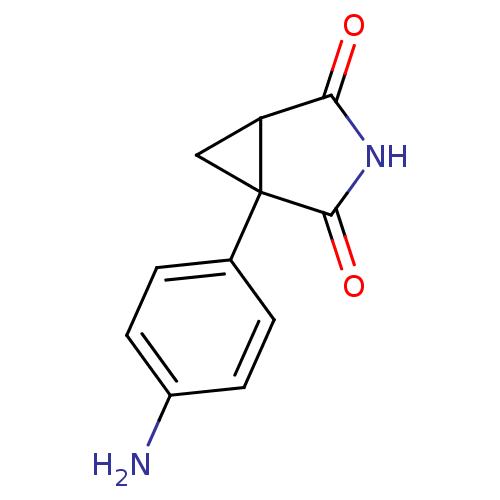
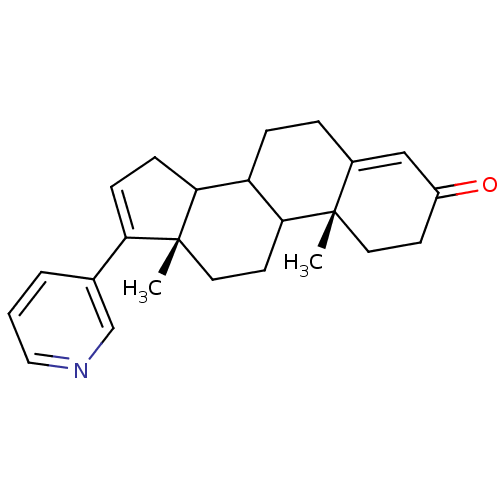
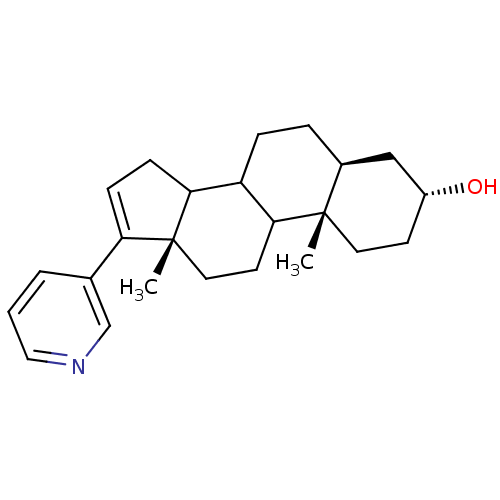
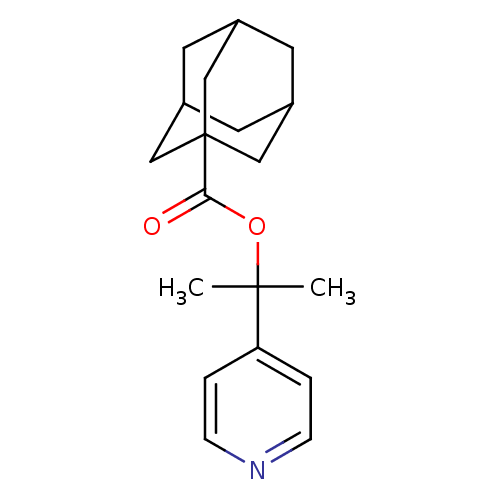
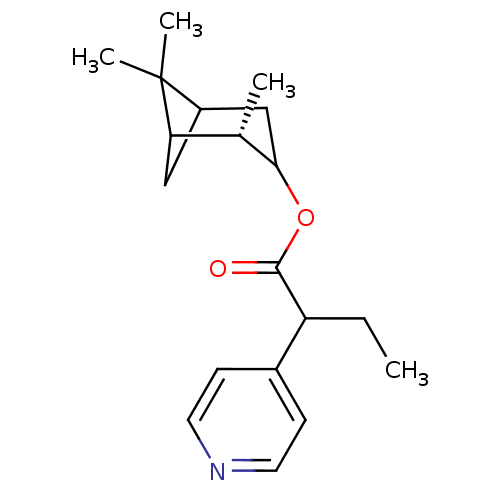
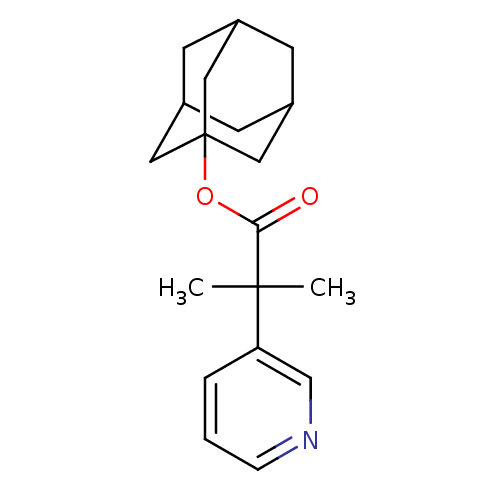
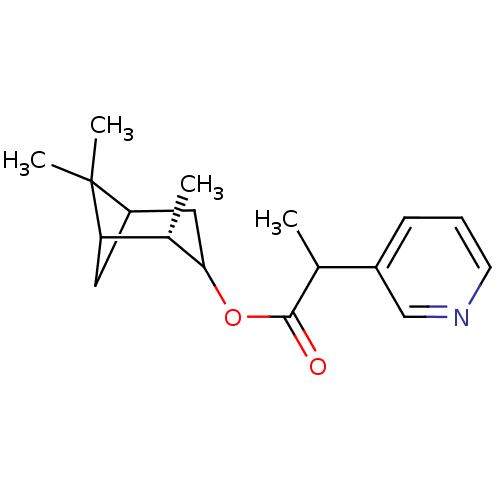
Affinity DataKi: 1.5nMAssay Description:Apparent inhibition constant (Ki) for cytochrome P450 19A1 with androstenedioneMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibition of human placental cytochrome P450 19A1 androstenedioneMore data for this Ligand-Target Pair
Affinity DataKi: 50nMAssay Description:Inhibition of human placental cytochrome P450 19A1 androstenedioneMore data for this Ligand-Target Pair
Affinity DataKi: 90nMAssay Description:Inhibition of human placental cytochrome P450 19A1 with androstenedioneMore data for this Ligand-Target Pair
Affinity DataKi: 120nMAssay Description:Inhibition of human placental cytochrome P450 19A1 with testosteroneMore data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:Inhibition of human placental cytochrome P450 19A1 with androstenedioneMore data for this Ligand-Target Pair
Affinity DataKi: 480nMAssay Description:Inhibition of human placental cytochrome P450 19A1 with androstenedioneMore data for this Ligand-Target Pair
Affinity DataKi: 600nMAssay Description:Inhibitory constant (Ki) for Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 600nMAssay Description:Inhibition of human placental aromatase Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 600nMAssay Description:Inhibitory activity against human placental cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nMAssay Description:Inhibitory constant (Ki) for Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nMAssay Description:Inhibition of human placental cytochrome P450 19A1 with androstenedioneMore data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nMAssay Description:Inhibition of human placental cytochrome P450 19A1 androstenedioneMore data for this Ligand-Target Pair
Affinity DataKi: 1.80E+3nMAssay Description:Inhibition of human placental cytochrome P450 19A1 with androstenedioneMore data for this Ligand-Target Pair
Affinity DataKi: 1.80E+3nMAssay Description:Inhibition of human placental cytochrome P450 19A1 with testosteroneMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1/2(Rattus norvegicus)
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataKi: 4.00E+3nMpH: 7.4Assay Description:Binding constant of Testosterone-5 alpha-reductase activity at pH 7.4More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1/2(Rattus norvegicus)
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataKi: 1.00E+4nMpH: 7.4Assay Description:Inhibition of Steroid 17-alpha-hydroxylase/17,20 lyase from rat testes microsomal preparationMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+4nMAssay Description:Inhibition of human placental cytochrome P450 19A1 with androstenedioneMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of C17,20-lyase enzyme, cytochrome P450 17A1 in Human testicular microsomesMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 2.5nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 2.60nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 2.70nMAssay Description:Inhibition of C17,20-lyase enzyme, cytochrome P450 17A1 in Human testicular microsomesMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 2.80nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 2.90nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 2.90nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of human progesterone 17-alpha-hydroxylase.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Inhibition of 17-alpha-hydroxylase enzyme, cytochrome P450 17A1 of human testicular microsomesMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human progesterone 17-alpha-hydroxylase.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 4.30nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 4.70nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Tested for inhibition of human testicular C17,20-Lyase.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human progesterone 17-alpha-hydroxylase.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 5.60nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Tested for inhibition of human testicular C17,20-Lyase.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Tested for inhibition of human testicular C17,20-Lyase.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 8.80nMAssay Description:Inhibition of 17-alpha-hydroxylase enzyme, cytochrome P450 17A1 of human testicular microsomesMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Tested for inhibition of human testicular C17,20-Lyase.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Tested for inhibition of human testicular C17,20-Lyase.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Tested for inhibition of human testicular C17,20-Lyase.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Tested for inhibition of human testicular C17,20-Lyase.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Tested for inhibition of human testicular C17,20-Lyase.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Tested for inhibition of human testicular C17,20-Lyase.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of human progesterone 17-alpha-hydroxylase.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Tested for inhibition of human testicular C17,20-Lyase.More data for this Ligand-Target Pair






 3D Structure (crystal)
3D Structure (crystal)