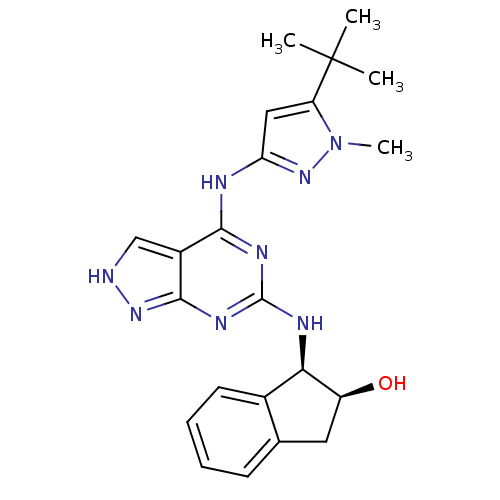
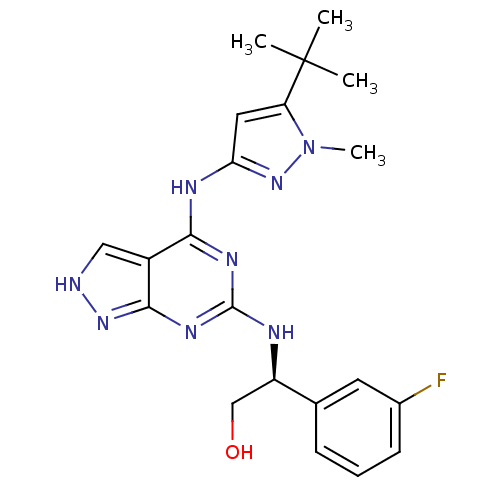
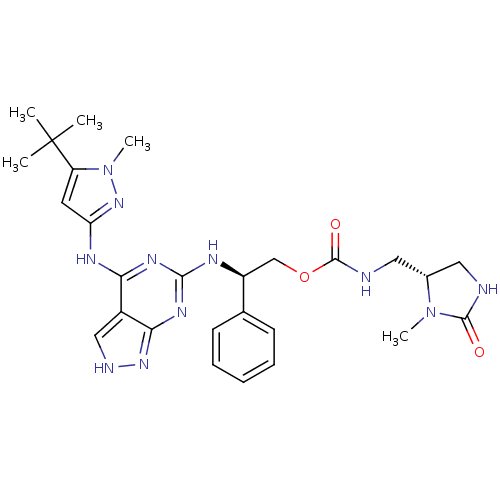
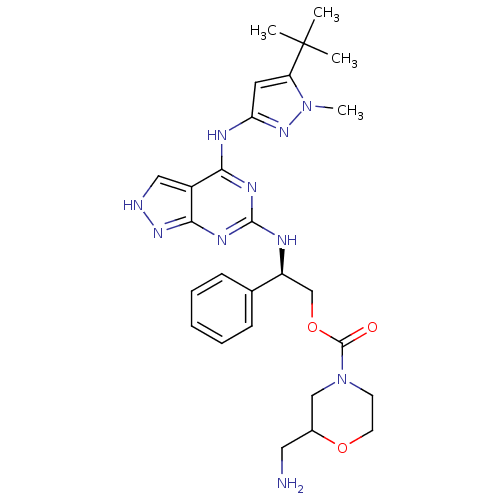
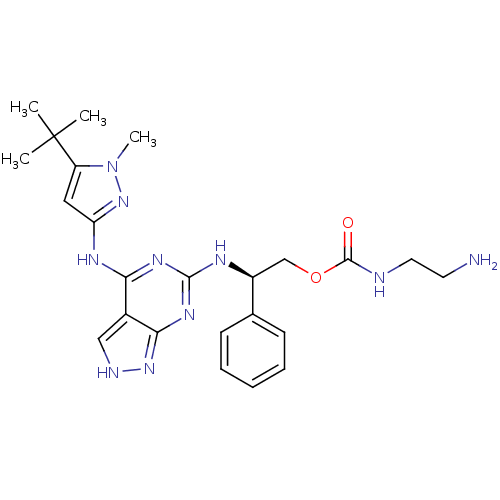
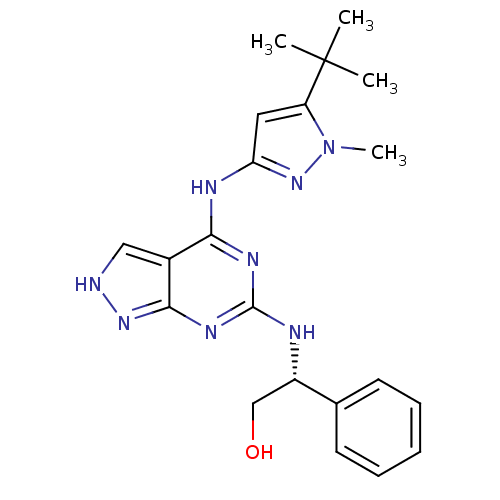
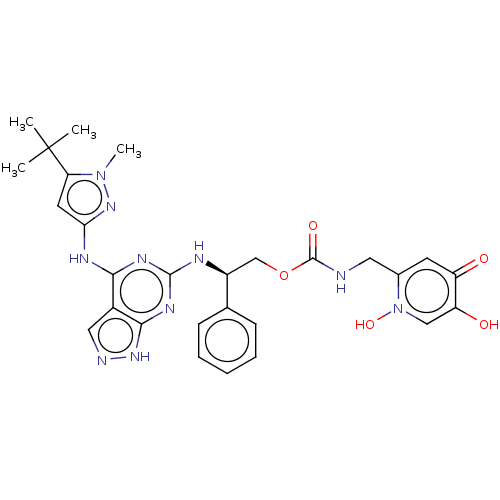
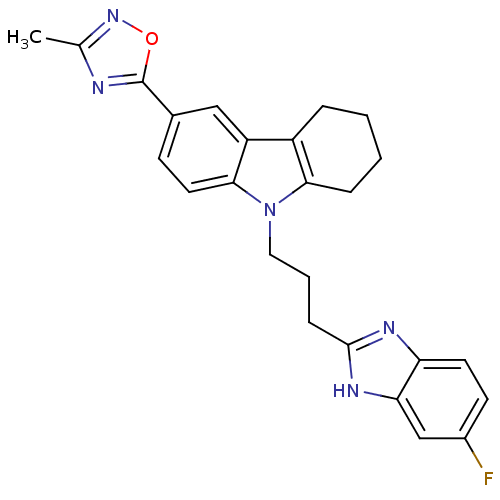
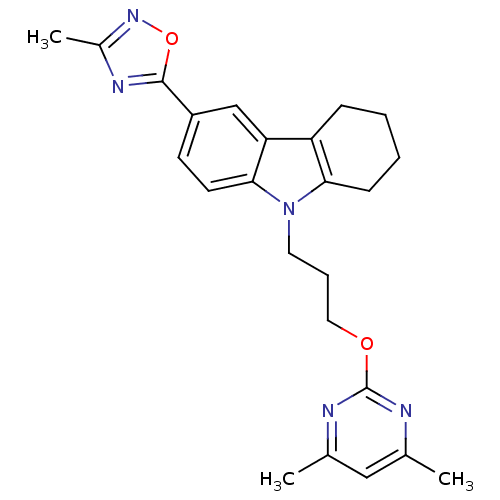
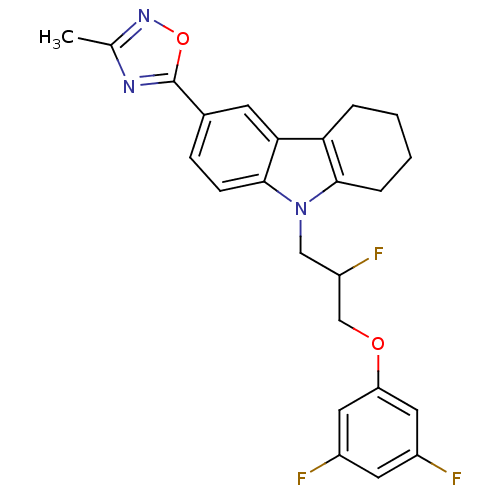
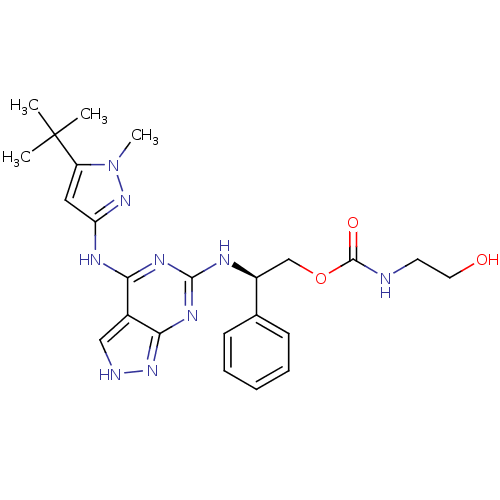
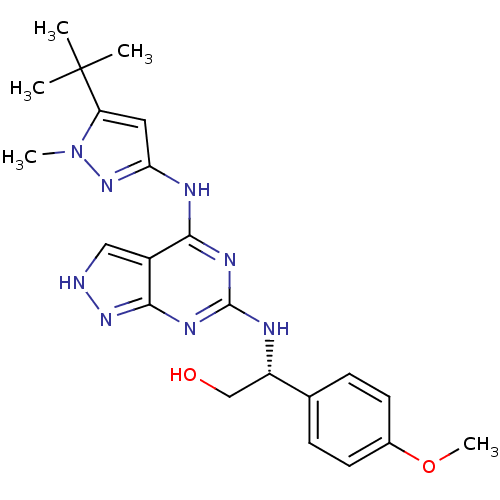
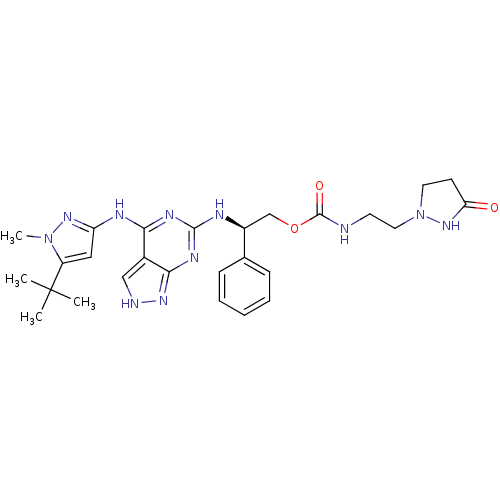
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Guru Nanak Dev University
Curated by ChEMBL
Guru Nanak Dev University
Curated by ChEMBL
Affinity DataKi: 0.0417nMAssay Description:Mixed type inhibition of electric eel AChE assessed as inhibition constant using varying levels of acetylthiocholine as substrate by reciprocal Linew...More data for this Ligand-Target Pair
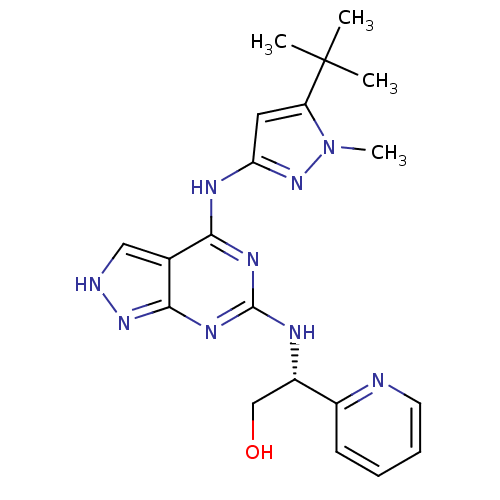
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: <0.5nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: <0.5nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: <0.5nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 0.600nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 0.600nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 0.700nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 1nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
Affinity DataIC50: <1nMT: 2°CAssay Description:The reactions (25 μL) were carried out in 25 mM Tris-HCl pH 8.0, 10 mM ammonium sulfate, 1.25 mM DTT, 0.002% Brij-35, 10 mM MgCl2, 40 μM UN...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 1.20nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 1.30nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetScavenger receptor class B member 1(Homo sapiens (Human))
Itherx Pharmaceuticals
Curated by ChEMBL
Itherx Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetScavenger receptor class B member 1(Homo sapiens (Human))
Itherx Pharmaceuticals
Curated by ChEMBL
Itherx Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetScavenger receptor class B member 1(Homo sapiens (Human))
Itherx Pharmaceuticals
Curated by ChEMBL
Itherx Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetScavenger receptor class B member 1(Homo sapiens (Human))
Itherx Pharmaceuticals
Curated by ChEMBL
Itherx Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetScavenger receptor class B member 1(Homo sapiens (Human))
Itherx Pharmaceuticals
Curated by ChEMBL
Itherx Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetScavenger receptor class B member 1(Homo sapiens (Human))
Itherx Pharmaceuticals
Curated by ChEMBL
Itherx Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetScavenger receptor class B member 1(Homo sapiens (Human))
Itherx Pharmaceuticals
Curated by ChEMBL
Itherx Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetScavenger receptor class B member 1(Homo sapiens (Human))
Itherx Pharmaceuticals
Curated by ChEMBL
Itherx Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetScavenger receptor class B member 1(Homo sapiens (Human))
Itherx Pharmaceuticals
Curated by ChEMBL
Itherx Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetScavenger receptor class B member 1(Homo sapiens (Human))
Itherx Pharmaceuticals
Curated by ChEMBL
Itherx Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetScavenger receptor class B member 1(Homo sapiens (Human))
Itherx Pharmaceuticals
Curated by ChEMBL
Itherx Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetScavenger receptor class B member 1(Homo sapiens (Human))
Itherx Pharmaceuticals
Curated by ChEMBL
Itherx Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetScavenger receptor class B member 1(Homo sapiens (Human))
Itherx Pharmaceuticals
Curated by ChEMBL
Itherx Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetScavenger receptor class B member 1(Homo sapiens (Human))
Itherx Pharmaceuticals
Curated by ChEMBL
Itherx Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human SRB1-mediated Hepatitis C virus genotype 1a entry into human HuH7.5 cells by immunoblottingMore data for this Ligand-Target Pair
TargetScavenger receptor class B member 1(Homo sapiens (Human))
Itherx Pharmaceuticals
Curated by ChEMBL
Itherx Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetScavenger receptor class B member 1(Homo sapiens (Human))
Itherx Pharmaceuticals
Curated by ChEMBL
Itherx Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 2nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMT: 2°CAssay Description:The reactions (25 μL) were carried out in 25 mM Tris-HCl pH 8.0, 10 mM ammonium sulfate, 1.25 mM DTT, 0.002% Brij-35, 10 mM MgCl2, 40 μM UN...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMT: 2°CAssay Description:The reactions (25 μL) were carried out in 25 mM Tris-HCl pH 8.0, 10 mM ammonium sulfate, 1.25 mM DTT, 0.002% Brij-35, 10 mM MgCl2, 40 μM UN...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMT: 2°CAssay Description:The reactions (25 μL) were carried out in 25 mM Tris-HCl pH 8.0, 10 mM ammonium sulfate, 1.25 mM DTT, 0.002% Brij-35, 10 mM MgCl2, 40 μM UN...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 4nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMT: 2°CAssay Description:The reactions (25 μL) were carried out in 25 mM Tris-HCl pH 8.0, 10 mM ammonium sulfate, 1.25 mM DTT, 0.002% Brij-35, 10 mM MgCl2, 40 μM UN...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMT: 2°CAssay Description:The reactions (25 μL) were carried out in 25 mM Tris-HCl pH 8.0, 10 mM ammonium sulfate, 1.25 mM DTT, 0.002% Brij-35, 10 mM MgCl2, 40 μM UN...More data for this Ligand-Target Pair
Affinity DataIC50: <5nMT: 2°CAssay Description:The reactions (25 μL) were carried out in 25 mM Tris-HCl pH 8.0, 10 mM ammonium sulfate, 1.25 mM DTT, 0.002% Brij-35, 10 mM MgCl2, 40 μM UN...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 5nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetScavenger receptor class B member 1(Homo sapiens (Human))
Itherx Pharmaceuticals
Curated by ChEMBL
Itherx Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMT: 2°CAssay Description:The reactions (25 μL) were carried out in 25 mM Tris-HCl pH 8.0, 10 mM ammonium sulfate, 1.25 mM DTT, 0.002% Brij-35, 10 mM MgCl2, 40 μM UN...More data for this Ligand-Target Pair
TargetScavenger receptor class B member 1(Homo sapiens (Human))
Itherx Pharmaceuticals
Curated by ChEMBL
Itherx Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetScavenger receptor class B member 1(Homo sapiens (Human))
Itherx Pharmaceuticals
Curated by ChEMBL
Itherx Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 6nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 7nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 7nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 8nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 8nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 8nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 8nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 9nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 10nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair
TargetUDP-N-acetylmuramate--L-alanine ligase(Pseudomonas aeruginosa (G-proteobacteria))
Astrazeneca India
Astrazeneca India
Affinity DataIC50: 10nMT: 2°CAssay Description:The reactions (50 μL) were carried out in 50 mM Tris-HCl pH 8.0, 20 mM ammonium sulfate, 2.5 mM DTT, 0.002% Brij-35, 1 mM MgCl2, 18 μM UNAM...More data for this Ligand-Target Pair