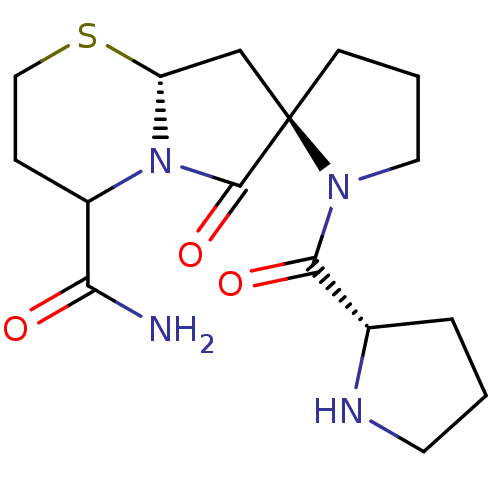
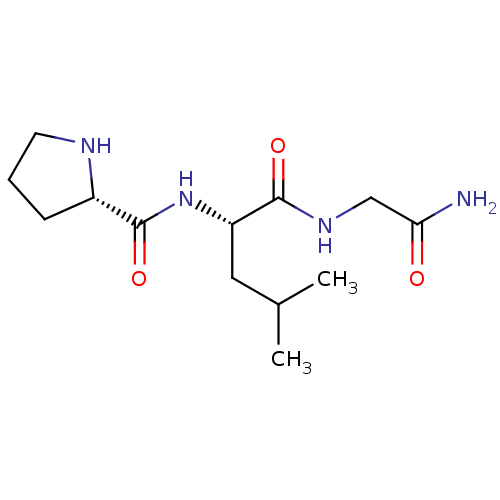
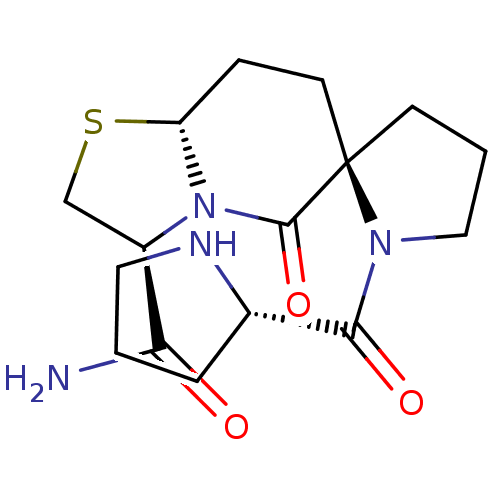
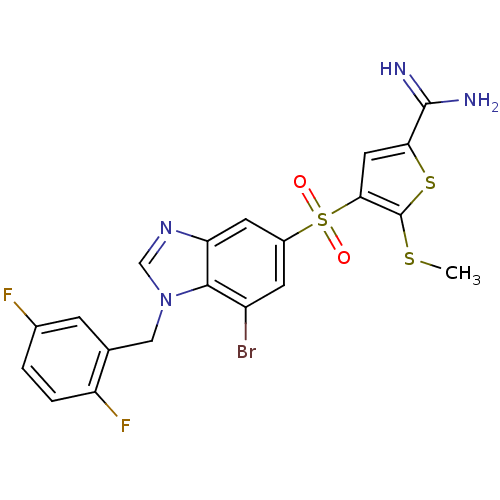
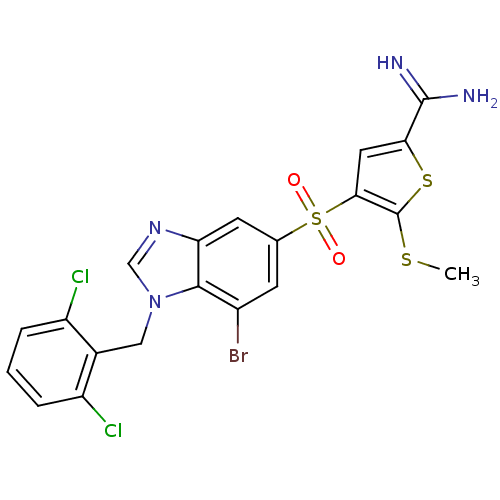
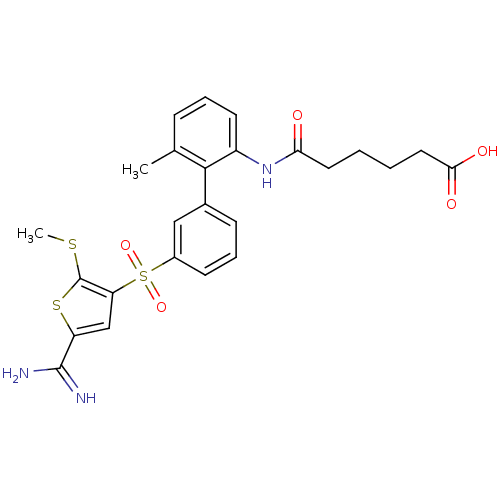
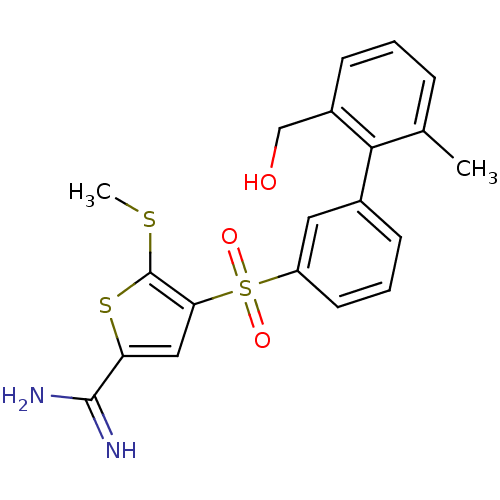
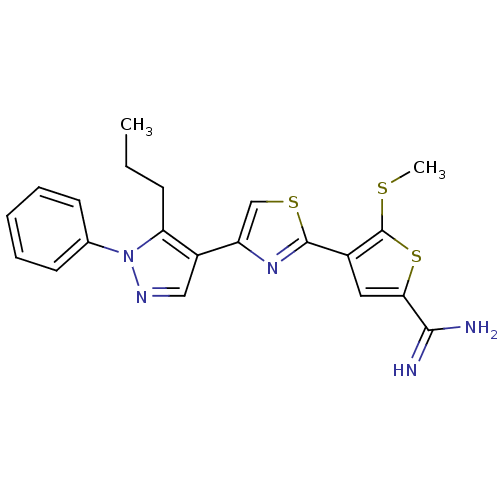
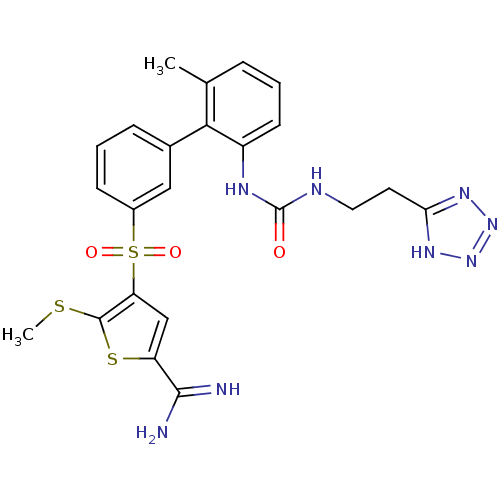
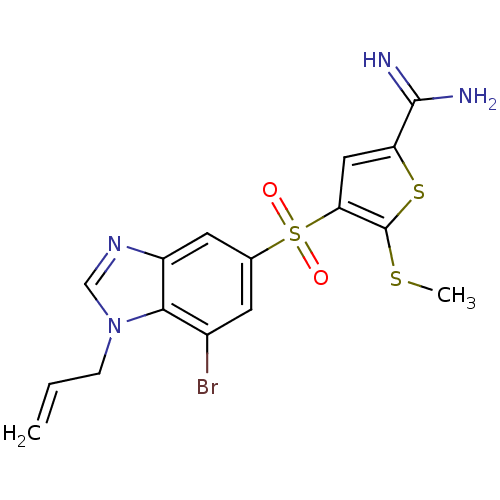
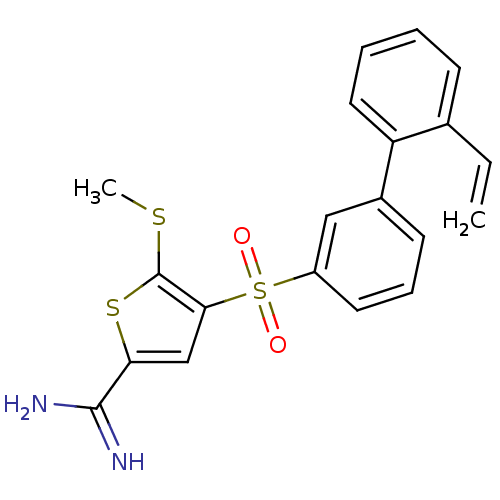
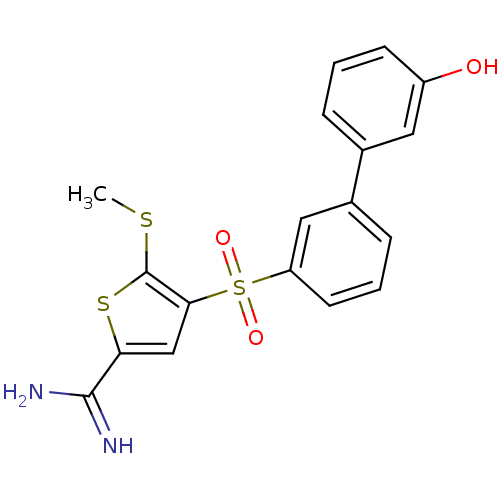
Affinity DataKi: 0.0310nMAssay Description:Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ...More data for this Ligand-Target Pair
Affinity DataKi: 0.0400nMAssay Description:Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ...More data for this Ligand-Target Pair
Affinity DataKi: 0.0500nMAssay Description:Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ...More data for this Ligand-Target Pair
Affinity DataKi: 0.0600nMAssay Description:Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of...More data for this Ligand-Target Pair
Affinity DataKi: 0.0640nMAssay Description:Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of...More data for this Ligand-Target Pair
Affinity DataKi: 0.0670nMAssay Description:Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ...More data for this Ligand-Target Pair
Affinity DataKi: 0.0710nMAssay Description:Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ...More data for this Ligand-Target Pair
Affinity DataKi: 0.0900nMAssay Description:Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of...More data for this Ligand-Target Pair
Affinity DataKi: 0.0900nMAssay Description:Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of ...More data for this Ligand-Target Pair
Affinity DataKi: 0.122nMAssay Description:Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of...More data for this Ligand-Target Pair
Affinity DataKi: 0.130nMAssay Description:Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of...More data for this Ligand-Target Pair
Affinity DataKi: 0.196nMAssay Description:Inhibitor constant of compound for high affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of...More data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 22nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 30nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 42nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 50nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:In vitro binding affinity towards human Complement C1s subcomponentMore data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Inhibitor constant of compound for low affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of ...More data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Inhibitor constant of compound for low affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in presence of ...More data for this Ligand-Target Pair
Affinity DataKi: 60.1nMAssay Description:Inhibitor constant of compound for low affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of G...More data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 64nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 70nMAssay Description:In vitro binding affinity towards human Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 73.2nMAssay Description:Inhibitor constant of compound for low affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of G...More data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 90nMAssay Description:In vitro binding affinity towards human Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 120nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 120nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 140nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 150nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 150nMAssay Description:In vitro binding affinity towards human Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 150nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 168nMAssay Description:Inhibitor constant of compound for low affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of G...More data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 180nMAssay Description:In vitro binding affinity towards trypsin was determinedMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 188nMAssay Description:Inhibitor constant of compound for low affinity component of [3H]-spiroperidol/N-propylnorapomorphine binding to Dopamine receptor D2 in absence of G...More data for this Ligand-Target Pair