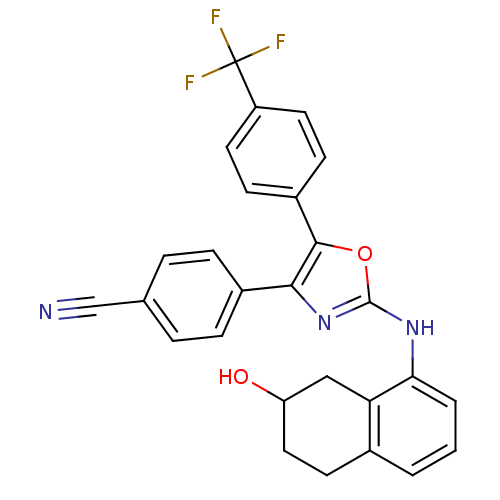
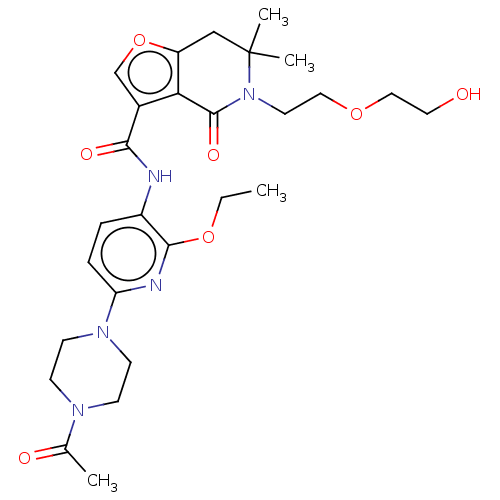
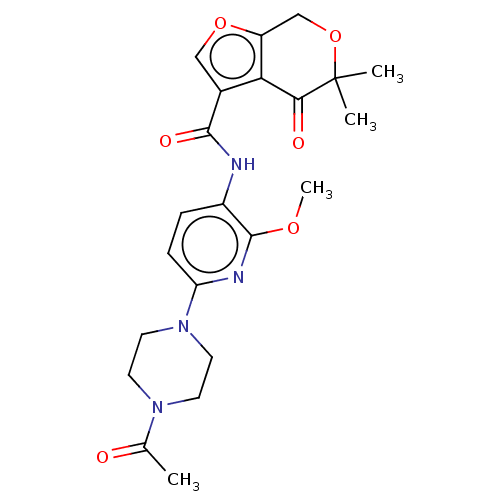
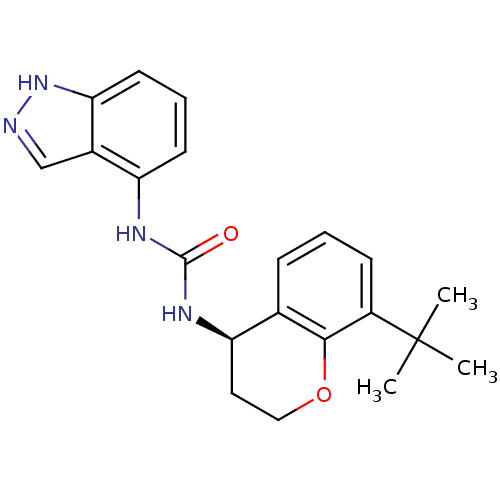
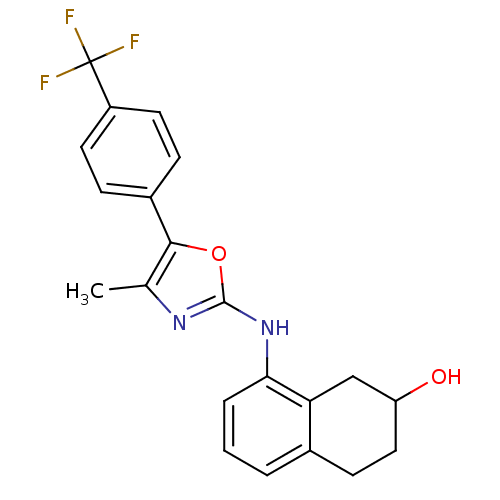
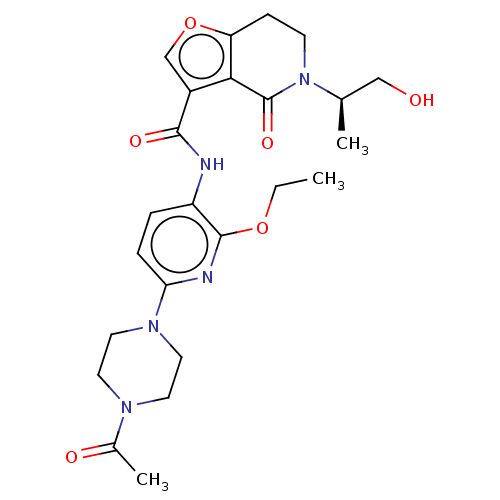
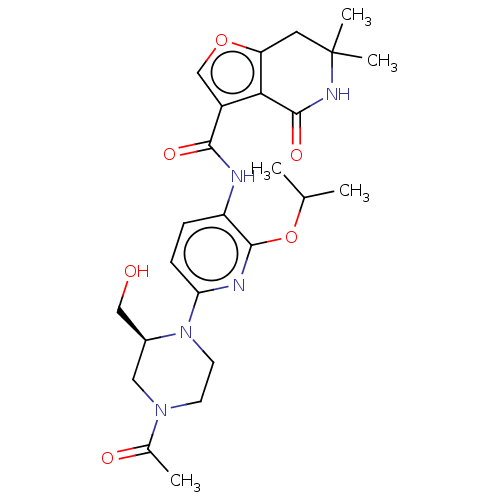
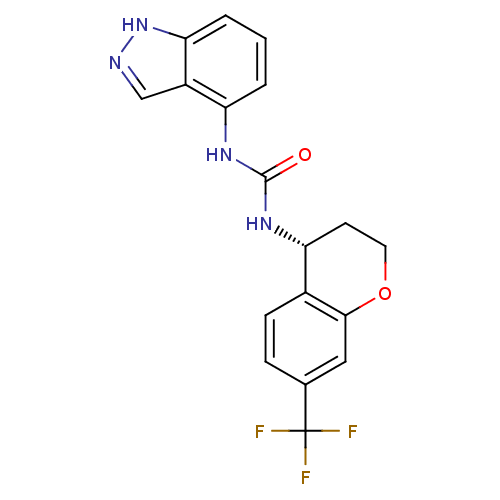
Affinity DataKi: 1.45nMAssay Description:Displacement of [3H]-histamine from rat histamine H4 receptor after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.51nMAssay Description:Displacement of [3H]-histamine from human histamine H4 receptor expressed in HEK293 cells after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [3H]-histamine from human histamine H4 receptor expressed in HEK293 cells after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2.63nMAssay Description:Displacement of [3H]-histamine from rat histamine H4 receptor after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2.69nMAssay Description:Displacement of [3H]-histamine from rat histamine H4 receptor after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 3.24nMAssay Description:Displacement of [3H]-histamine from rat histamine H4 receptor after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 4.47nMAssay Description:Displacement of [3H]-histamine from rat histamine H4 receptor after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 6.76nMAssay Description:Displacement of [3H]NAMH from rat histamine H3 receptor expressed in C6 cells after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 7.08nMAssay Description:Displacement of [3H]-histamine from human histamine H4 receptor expressed in HEK293 cells after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 39.8nMAssay Description:Displacement of [3H]-histamine from human histamine H4 receptor expressed in HEK293 cells after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 49.0nMAssay Description:Displacement of [3H]-histamine from human histamine H4 receptor expressed in HEK293 cells after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 63.1nMAssay Description:Displacement of [3H]NAMH from human histamine H3 receptor expressed in C6 cells after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 72.4nMAssay Description:Displacement of [3H]NAMH from rat histamine H3 receptor expressed in C6 cells after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 79.4nMAssay Description:Displacement of [3H]NAMH from rat histamine H3 receptor expressed in C6 cells after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 105nMAssay Description:Displacement of [3H]NAMH from human histamine H3 receptor expressed in C6 cells after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 105nMAssay Description:Displacement of [3H]NAMH from rat histamine H3 receptor expressed in C6 cells after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 224nMAssay Description:Displacement of [3H]NAMH from human histamine H3 receptor expressed in C6 cells after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 295nMAssay Description:Displacement of [3H]NAMH from human histamine H3 receptor expressed in C6 cells after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 661nMAssay Description:Displacement of [3H]NAMH from rat histamine H3 receptor expressed in C6 cells after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2.24E+3nMAssay Description:Displacement of [3H]NAMH from human histamine H3 receptor expressed in C6 cells after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:In vitro concentration required for 50% inhibition against Adenosine Kinase (AK) in the presence of intact cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:In vitro concentration required for 50% inhibition against Adenosine Kinase (AK) in the presence of enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:In vitro concentration required for 50% inhibition against Adenosine Kinase (AK) in the presence of enzymeMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:Inhibitory concentration against adenosine kinase was determinedMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Antagonist activity at human TRPV1 assessed as inhibition of calcium influxMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Antagonist activity at human TRPV1 assessed as inhibition of calcium influxMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:Antagonist activity at human recombinant TRPV1 receptor expressed in human 1321 cells assessed as inhibition of capsaicin-induced in intracellular ca...More data for this Ligand-Target Pair
Affinity DataIC50: 3.40nMAssay Description:In vitro concentration required for 50% inhibition against Adenosine Kinase (AK) in the presence of intact cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 3.5nMAssay Description:Antagonist activity at human TRPV1More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Antagonist activity at human TRPV1 assessed as inhibition of calcium influxMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Antagonist activity at human TRPV1 assessed as inhibition of calcium influxMore data for this Ligand-Target Pair
Affinity DataIC50: 4.10nMAssay Description:In vitro concentration required for 50% inhibition against Adenosine Kinase (AK) in the presence of enzymeMore data for this Ligand-Target Pair