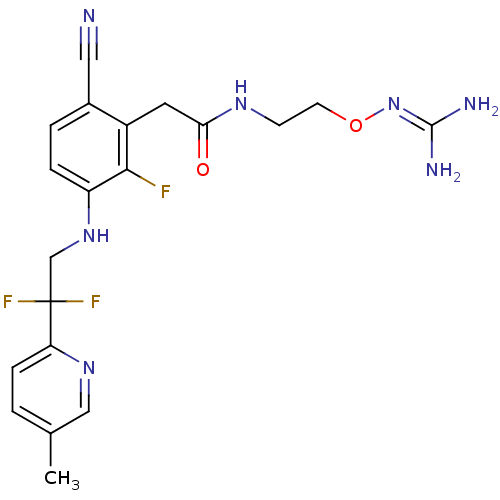
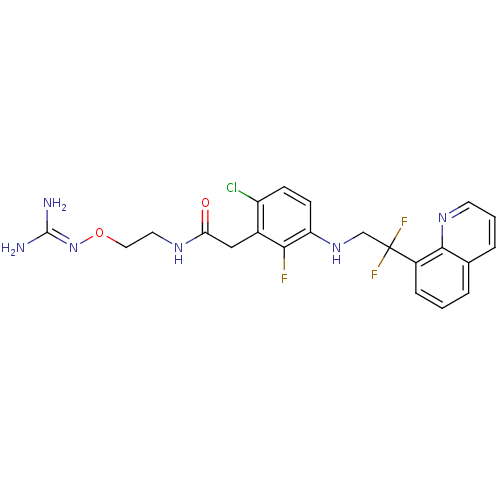
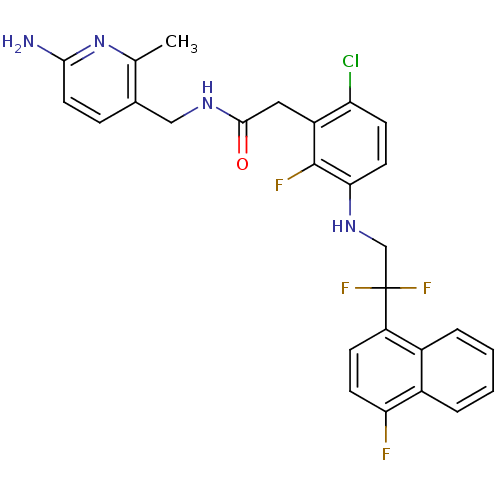
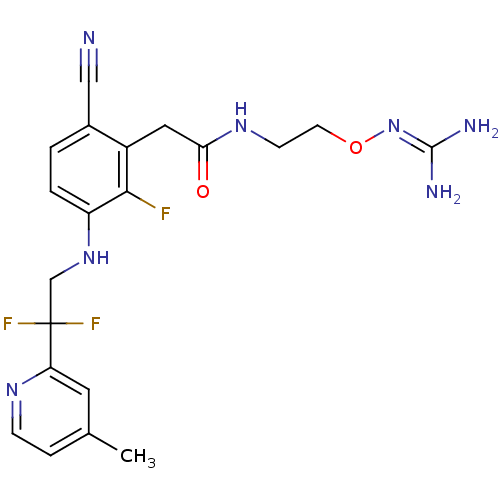
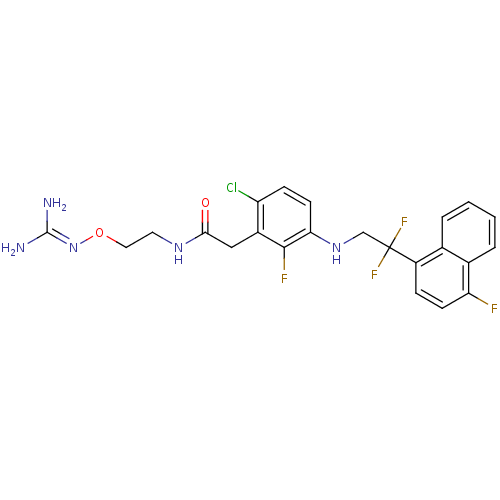
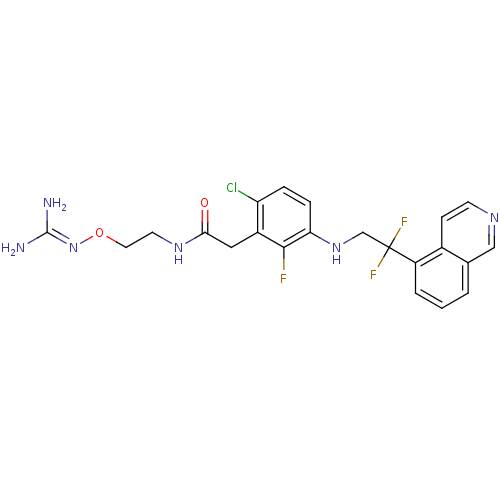
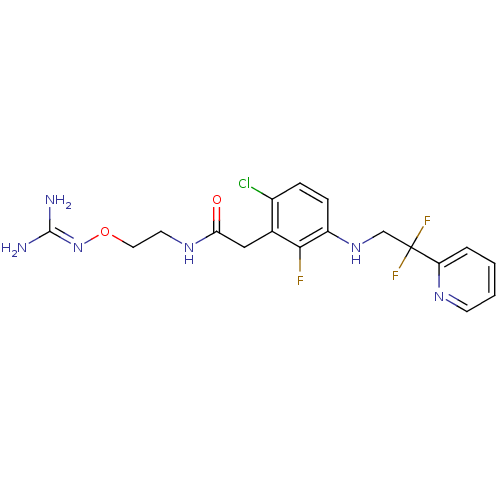
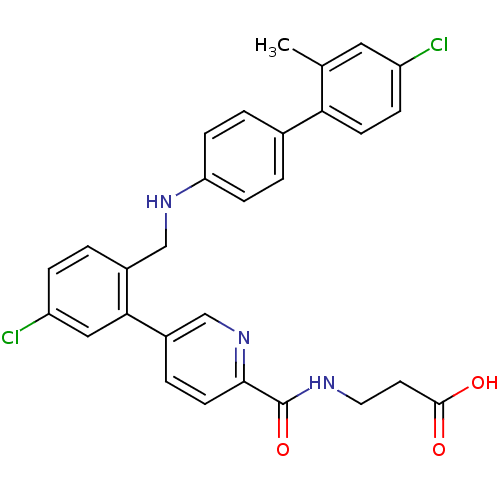
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.380nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.570nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.650nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.660nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Inhibition of human thrombin after 15 mins by standard chromogenic assayMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.770nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.790nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.900nMAssay Description:Inhibition of human thrombin after 15 mins by standard chromogenic assayMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.80nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.80nMAssay Description:Inhibition of human thrombin after 15 mins by standard chromogenic assayMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.30nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.70nMAssay Description:Inhibition of human thrombin after 15 mins by standard chromogenic assayMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.80nMAssay Description:Inhibition of human thrombin after 15 mins by standard chromogenic assayMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.90nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.10nMAssay Description:Inhibition of human thrombin after 15 mins by standard chromogenic assayMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.20nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.30nMAssay Description:Inhibition of human thrombin after 15 mins by standard chromogenic assayMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Binding affinity to thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4.70nMAssay Description:Inhibition of human thrombin after 15 mins by standard chromogenic assayMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 8.60nMAssay Description:Inhibition of human thrombin after 15 mins by standard chromogenic assayMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataKi: 9.20nMAssay Description:Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con...More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Inhibition of human thrombin after 15 mins by standard chromogenic assayMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con...More data for this Ligand-Target Pair
Affinity DataKi: 12.4nMpH: 7.4Assay Description:The binding assay was performed by a filtration method in a 384 well format. Membranes at a final protein concentration of 6 μg/well were incuba...More data for this Ligand-Target Pair
Affinity DataKi: 14.4nMpH: 7.4Assay Description:The binding assay was performed by a filtration method in a 384 well format. Membranes at a final protein concentration of 6 μg/well were incuba...More data for this Ligand-Target Pair
Affinity DataKi: 14.9nMpH: 7.4Assay Description:The binding assay was performed by a filtration method in a 384 well format. Membranes at a final protein concentration of 6 μg/well were incuba...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataKi: 15nMAssay Description:Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con...More data for this Ligand-Target Pair
Affinity DataKi: 15.7nMpH: 7.4Assay Description:The binding assay was performed by a filtration method in a 384 well format. Membranes at a final protein concentration of 6 μg/well were incuba...More data for this Ligand-Target Pair
Affinity DataKi: 16.5nMpH: 7.4Assay Description:The binding assay was performed by a filtration method in a 384 well format. Membranes at a final protein concentration of 6 μg/well were incuba...More data for this Ligand-Target Pair
Affinity DataKi: 16.5nMpH: 7.4Assay Description:The binding assay was performed by a filtration method in a 384 well format. Membranes at a final protein concentration of 6 μg/well were incuba...More data for this Ligand-Target Pair
Affinity DataKi: 16.8nMpH: 7.4Assay Description:The binding assay was performed by a filtration method in a 384 well format. Membranes at a final protein concentration of 6 μg/well were incuba...More data for this Ligand-Target Pair
Affinity DataKi: 17.2nMpH: 7.4Assay Description:The binding assay was performed by a filtration method in a 384 well format. Membranes at a final protein concentration of 6 μg/well were incuba...More data for this Ligand-Target Pair
Affinity DataKi: 18.3nMpH: 7.4Assay Description:The binding assay was performed by a filtration method in a 384 well format. Membranes at a final protein concentration of 6 μg/well were incuba...More data for this Ligand-Target Pair
Affinity DataKi: 18.4nMpH: 7.4Assay Description:The binding assay was performed by a filtration method in a 384 well format. Membranes at a final protein concentration of 6 μg/well were incuba...More data for this Ligand-Target Pair
Affinity DataKi: 18.7nMpH: 7.4Assay Description:The binding assay was performed by a filtration method in a 384 well format. Membranes at a final protein concentration of 6 μg/well were incuba...More data for this Ligand-Target Pair
Affinity DataKi: 18.8nMpH: 7.4Assay Description:The binding assay was performed by a filtration method in a 384 well format. Membranes at a final protein concentration of 6 μg/well were incuba...More data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 19nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 19.3nMpH: 7.4Assay Description:The binding assay was performed by a filtration method in a 384 well format. Membranes at a final protein concentration of 6 μg/well were incuba...More data for this Ligand-Target Pair
Affinity DataKi: 19.4nMpH: 7.4Assay Description:The binding assay was performed by a filtration method in a 384 well format. Membranes at a final protein concentration of 6 μg/well were incuba...More data for this Ligand-Target Pair










 3D Structure (crystal)
3D Structure (crystal)