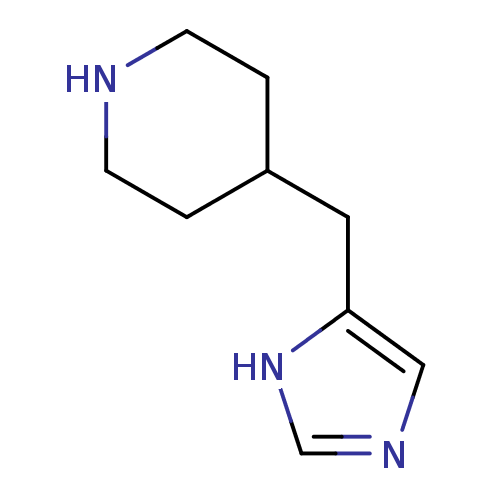
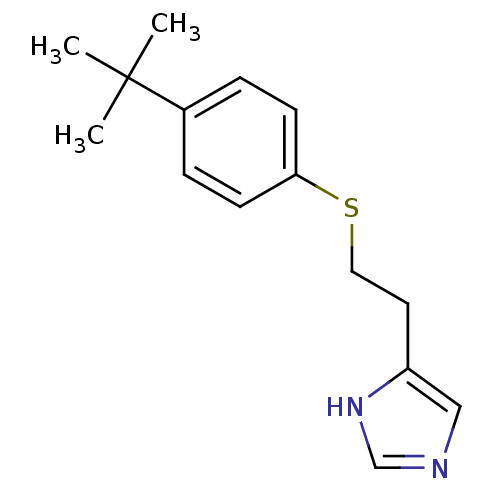
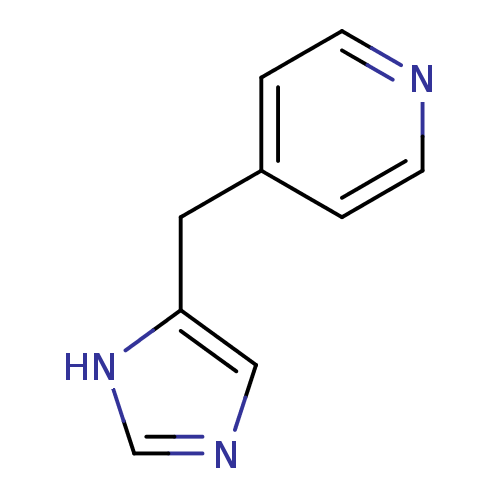
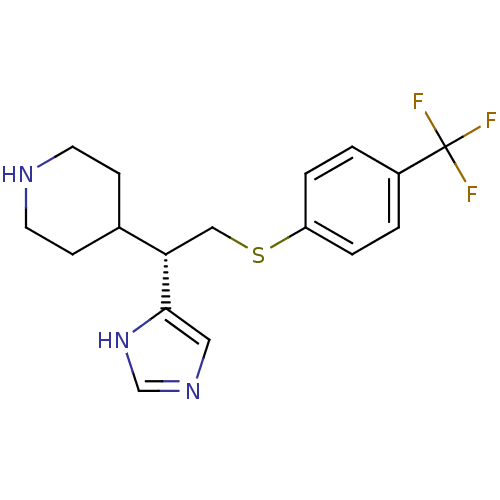
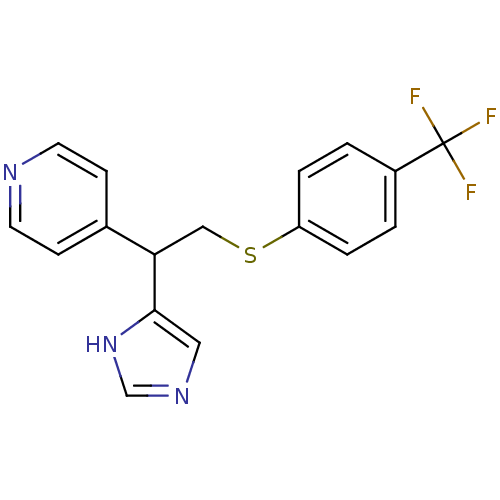
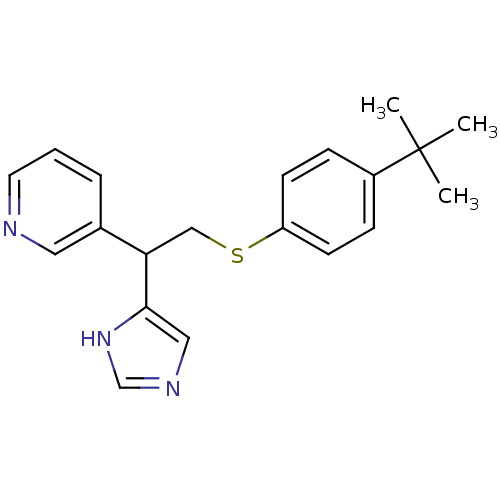
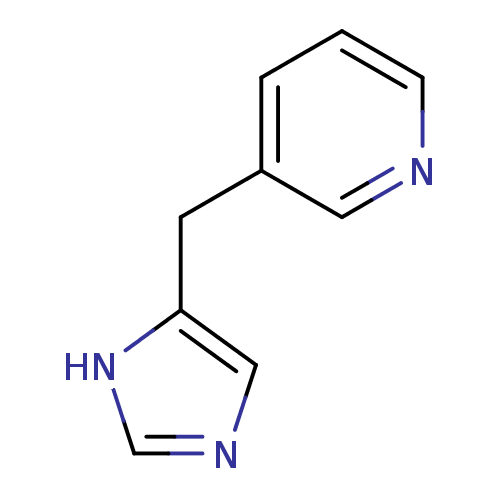
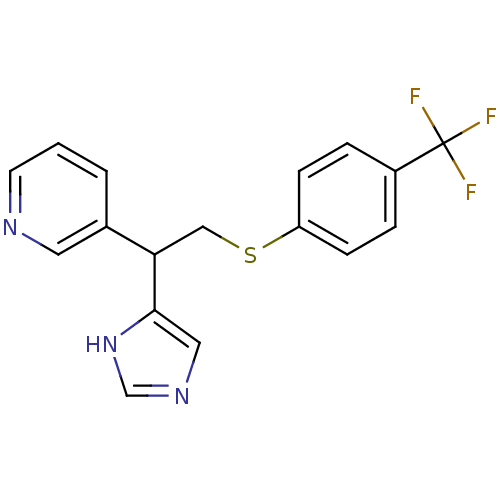
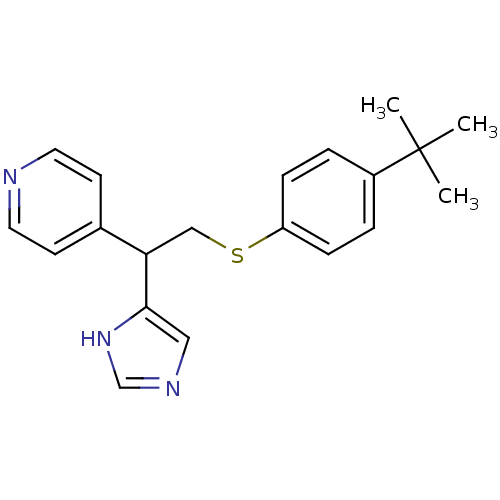
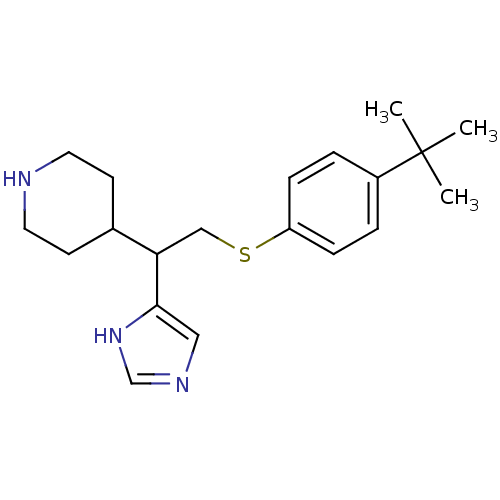
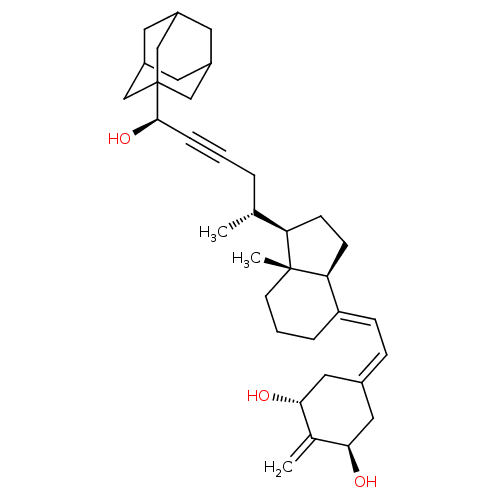
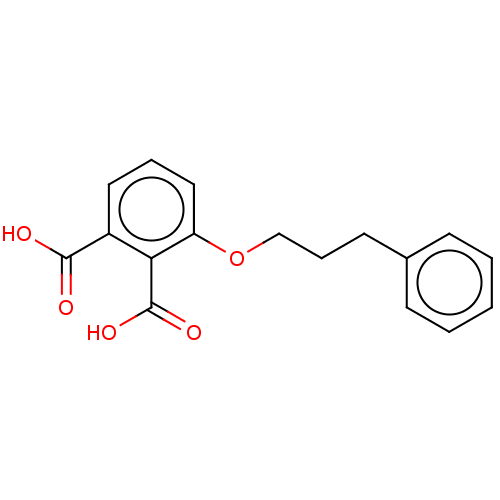
Affinity DataKi: 0.300nMAssay Description:Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.430nMAssay Description:Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.770nMAssay Description:Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 17nMAssay Description:Binding affinity to human histamine H4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 48nMAssay Description:Binding affinity to human histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 64nMAssay Description:Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 75nMAssay Description:Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 76nMAssay Description:Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 120nMAssay Description:Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 180nMAssay Description:Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 280nMAssay Description:Binding affinity to human histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 310nMAssay Description:Binding affinity to human histamine H4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 360nMAssay Description:Binding affinity to human histamine H4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 530nMAssay Description:Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 540nMAssay Description:Binding affinity to human histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 840nMAssay Description:Binding affinity to human histamine H4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.30E+3nMAssay Description:Binding affinity to human histamine H4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.80E+3nMAssay Description:Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity to human histamine H2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity to human histamine H2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity to human histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity to human histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity to human histamine H2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity to human histamine H2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity to human histamine H2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Displacement of [3H]-1,25(OH)2D3 from GST-fused human VDR-LBD expressed in Escherichia coli BL21More data for this Ligand-Target Pair
Affinity DataIC50: 8.5nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Displacement of [3H]-1,25(OH)2D3 from GST-fused human VDR-LBD expressed in Escherichia coli BL21More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Displacement of [3H]-1,25(OH)2D3 from GST-fused human VDR-LBD expressed in Escherichia coli BL21More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Displacement of [3H]-1,25(OH)2D3 from GST-fused human VDR-LBD expressed in Escherichia coli BL21More data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 1.55E+3nMAssay Description:Inhibition of carbapenems-resistant Pseudomonas aeruginosa MSC15369 metallo-beta-lactamase IMP1 expressed in Escherichia coli DH5[alpha] using nitroc...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of carbapenems-resistant Pseudomonas aeruginosa MSC15369 metallo-beta-lactamase IMP1 expressed in Escherichia coli DH5[alpha] using nitroc...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of carbapenems-resistant Pseudomonas aeruginosa MSC15369 metallo-beta-lactamase IMP1 expressed in Escherichia coli DH5[alpha] using nitroc...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of carbapenems-resistant Pseudomonas aeruginosa MSC15369 metallo-beta-lactamase IMP1 expressed in Escherichia coli DH5[alpha] using nitroc...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of carbapenems-resistant Pseudomonas aeruginosa MSC15369 metallo-beta-lactamase IMP1 expressed in Escherichia coli DH5[alpha] using nitroc...More data for this Ligand-Target Pair