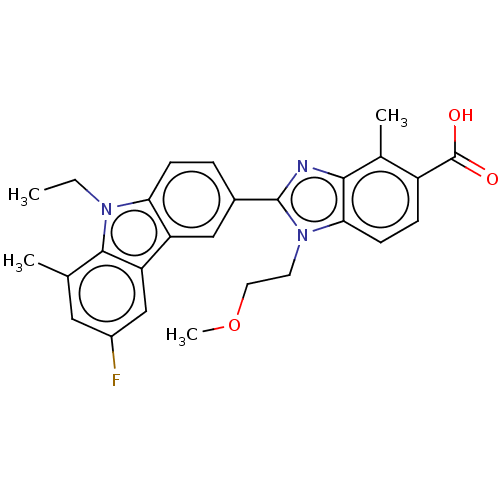
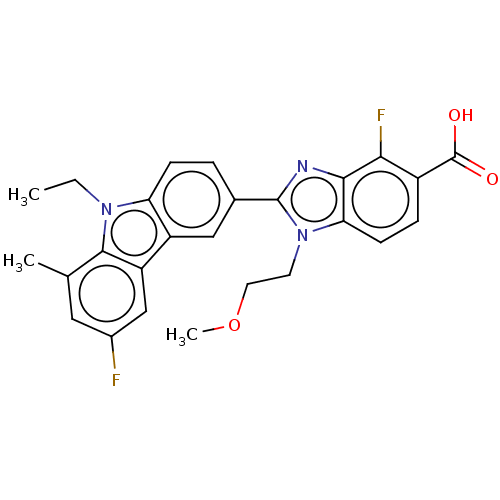
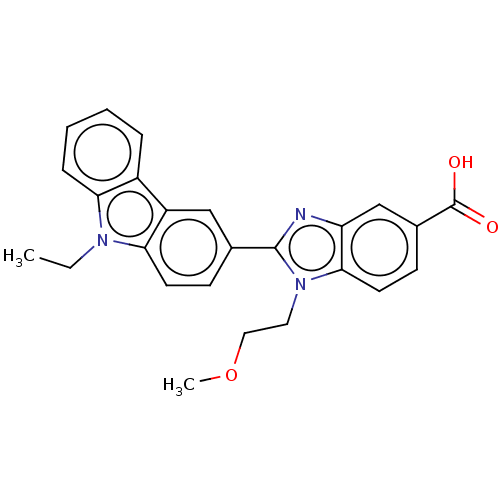
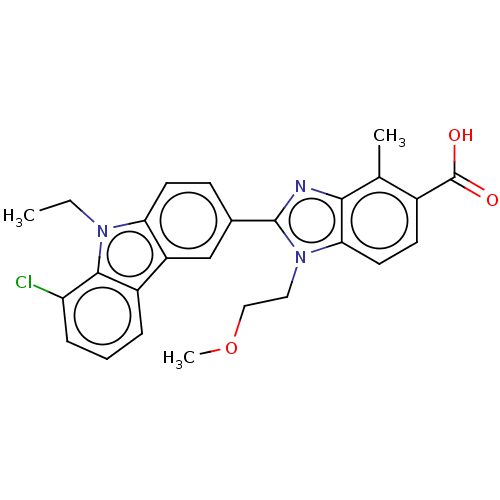
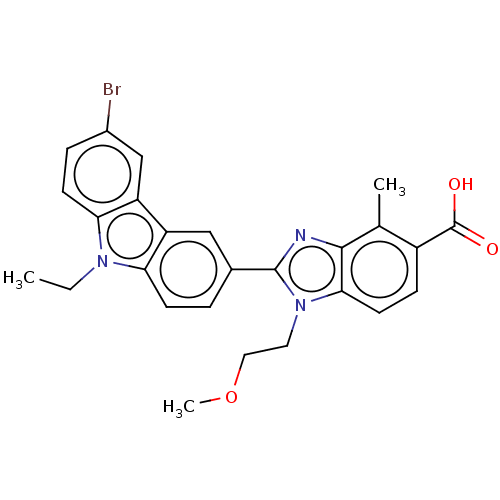
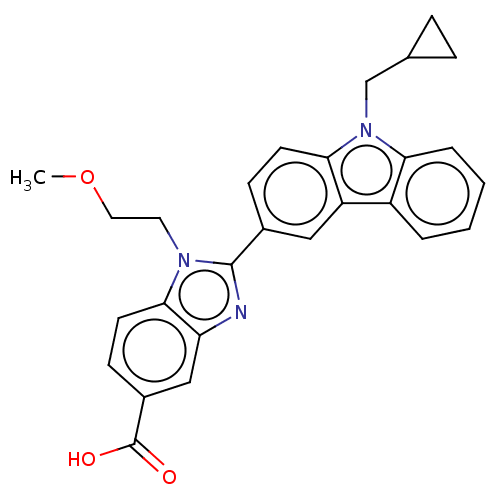
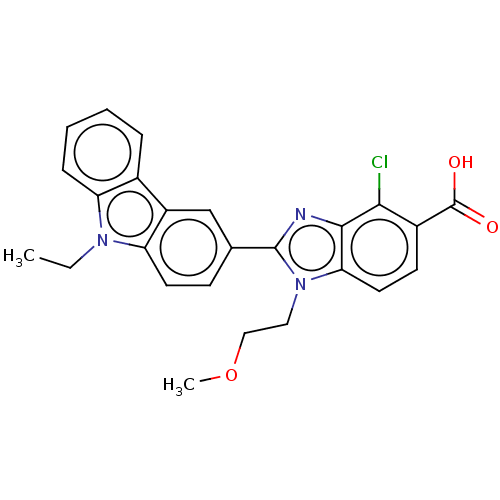
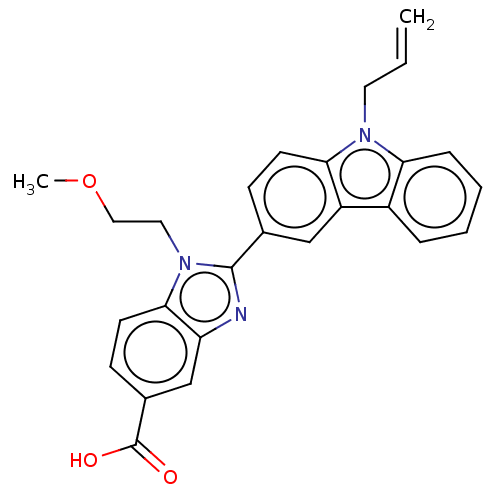
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 1.5nMAssay Description:5. 1 Detection PrincipleBinding of prostaglandin D2 to the human PGD receptor induces activation of membrane-bound adenylate cyclases and leads to th...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 1.90nMAssay Description:5. 1 Detection PrincipleBinding of prostaglandin D2 to the human PGD receptor induces activation of membrane-bound adenylate cyclases and leads to th...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 2nMAssay Description:5. 1 Detection PrincipleBinding of prostaglandin D2 to the human PGD receptor induces activation of membrane-bound adenylate cyclases and leads to th...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 2.40nMAssay Description:5. 1 Detection PrincipleBinding of prostaglandin D2 to the human PGD receptor induces activation of membrane-bound adenylate cyclases and leads to th...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 2.40nMAssay Description:5. 1 Detection PrincipleBinding of prostaglandin D2 to the human PGD receptor induces activation of membrane-bound adenylate cyclases and leads to th...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 2.80nMAssay Description:5. 1 Detection PrincipleBinding of prostaglandin D2 to the human PGD receptor induces activation of membrane-bound adenylate cyclases and leads to th...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 4.16nMT: 2°CAssay Description:4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 4.20nMAssay Description:Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assayMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 4.30nMAssay Description:Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assayMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 4.40nMAssay Description:Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assayMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 4.70nMAssay Description:Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assayMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 5.19nMT: 2°CAssay Description:4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 5.20nMAssay Description:Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assayMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 5.59nMT: 2°CAssay Description:4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 6.10nMAssay Description:Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assayMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 6.36nMT: 2°CAssay Description:4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 6.42nMT: 2°CAssay Description:4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 6.80nMAssay Description:Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assayMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 7nMAssay Description:Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assayMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 7nMT: 2°CAssay Description:4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 10.2nMT: 2°CAssay Description:4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 10.3nMT: 2°CAssay Description:4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 11nMAssay Description:5. 1 Detection PrincipleBinding of prostaglandin D2 to the human PGD receptor induces activation of membrane-bound adenylate cyclases and leads to th...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 11.6nMT: 2°CAssay Description:4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 12.4nMT: 2°CAssay Description:4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 13nMAssay Description:Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 13.1nMT: 2°CAssay Description:4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 13.4nMT: 2°CAssay Description:4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 14.3nMT: 2°CAssay Description:4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 14.4nMT: 2°CAssay Description:4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 14.7nMT: 2°CAssay Description:4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 15nMAssay Description:Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assayMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 15nMAssay Description:Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assayMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 15.3nMT: 2°CAssay Description:4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 16nMAssay Description:Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of LHRH-induced response preincubated for 20 mins followe...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 16.1nMT: 2°CAssay Description:4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 16.5nMT: 2°CAssay Description:4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 16.9nMT: 2°CAssay Description:4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 17nMAssay Description:Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assayMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 17.6nMT: 2°CAssay Description:4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (...More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Antagonist activity at human GnRH receptor expressed in CHO-K1 cells assessed as inhibition of buserelin-induced response preincubated for 20 mins fo...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 18nMAssay Description:Antagonist activity at human EP4R assessed as inhibition of agonist-induced cAMP production by fluorescent cAMP tracer cAMP-d2 based FRET assayMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 18nMT: 2°CAssay Description:4 μl of a cAMP-d2/cell suspension (625000 cells/ml) were added to a test plate containing the substance solutions already initially introduced (...More data for this Ligand-Target Pair